当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells.
Nature Communications ( IF 14.7 ) Pub Date : 2016-09-19 , DOI: 10.1038/ncomms12897
Corina T. Madreiter-Sokolowski , Christiane Klec , Warisara Parichatikanond , Sarah Stryeck , Benjamin Gottschalk , Sergio Pulido , Rene Rost , Emrah Eroglu , Nicole A. Hofmann , Alexander I. Bondarenko , Tobias Madl , Markus Waldeck-Weiermair , Roland Malli , Wolfgang F. Graier
Nature Communications ( IF 14.7 ) Pub Date : 2016-09-19 , DOI: 10.1038/ncomms12897
Corina T. Madreiter-Sokolowski , Christiane Klec , Warisara Parichatikanond , Sarah Stryeck , Benjamin Gottschalk , Sergio Pulido , Rene Rost , Emrah Eroglu , Nicole A. Hofmann , Alexander I. Bondarenko , Tobias Madl , Markus Waldeck-Weiermair , Roland Malli , Wolfgang F. Graier
|
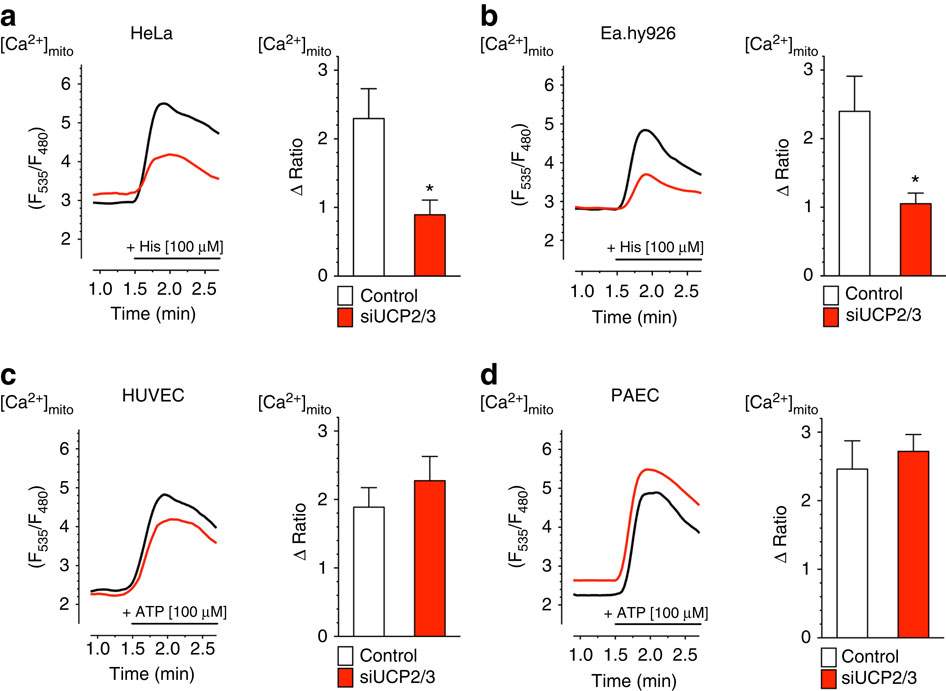
Recent studies revealed that mitochondrial Ca(2+) channels, which control energy flow, cell signalling and death, are macromolecular complexes that basically consist of the pore-forming mitochondrial Ca(2+) uniporter (MCU) protein, the essential MCU regulator (EMRE), and the mitochondrial Ca(2+) uptake 1 (MICU1). MICU1 is a regulatory subunit that shields mitochondria from Ca(2+) overload. Before the identification of these core elements, the novel uncoupling proteins 2 and 3 (UCP2/3) have been shown to be fundamental for mitochondrial Ca(2+) uptake. Here we clarify the molecular mechanism that determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake. Our data demonstrate that mitochondrial Ca(2+) uptake is controlled by protein arginine methyl transferase 1 (PRMT1) that asymmetrically methylates MICU1, resulting in decreased Ca(2+) sensitivity. UCP2/3 normalize Ca(2+) sensitivity of methylated MICU1 and, thus, re-establish mitochondrial Ca(2+) uptake activity. These data provide novel insights in the complex regulation of the mitochondrial Ca(2+) uniporter by PRMT1 and UCP2/3.
中文翻译:

PRMT1介导的MICU1甲基化确定永生化细胞中线粒体Ca(2+)摄取的UCP2 / 3依赖性。
最近的研究表明,控制能量流,细胞信号传导和死亡的线粒体Ca(2+)通道是大分子复合物,主要由形成孔的线粒体Ca(2+)单向转运蛋白(MCU)蛋白,必需的MCU调节剂( EMRE)和线粒体Ca(2+)摄取1(MICU1)。MICU1是一个调节亚基,可保护线粒体免受Ca(2+)超负荷的影响。在确定这些核心元素之前,新型的解偶联蛋白2和3(UCP2 / 3)已被证明是线粒体Ca(2+)吸收的基础。在这里,我们阐明了决定线粒体Ca(2+)吸收的UCP2 / 3依赖性的分子机制。我们的数据表明,线粒体Ca(2+)的摄取受蛋白质精氨酸甲基转移酶1(PRMT1)的控制,该蛋白质不对称地甲基化MICU1,导致Ca(2+)敏感性降低。UCP2 / 3标准化甲基化的MICU1的Ca(2+)敏感性,并因此重新建立线粒体Ca(2+)的吸收活性。这些数据提供了由PRMT1和UCP2 / 3对线粒体Ca(2+)单向转运体的复杂调控的新颖见解。
更新日期:2016-09-21
中文翻译:

PRMT1介导的MICU1甲基化确定永生化细胞中线粒体Ca(2+)摄取的UCP2 / 3依赖性。
最近的研究表明,控制能量流,细胞信号传导和死亡的线粒体Ca(2+)通道是大分子复合物,主要由形成孔的线粒体Ca(2+)单向转运蛋白(MCU)蛋白,必需的MCU调节剂( EMRE)和线粒体Ca(2+)摄取1(MICU1)。MICU1是一个调节亚基,可保护线粒体免受Ca(2+)超负荷的影响。在确定这些核心元素之前,新型的解偶联蛋白2和3(UCP2 / 3)已被证明是线粒体Ca(2+)吸收的基础。在这里,我们阐明了决定线粒体Ca(2+)吸收的UCP2 / 3依赖性的分子机制。我们的数据表明,线粒体Ca(2+)的摄取受蛋白质精氨酸甲基转移酶1(PRMT1)的控制,该蛋白质不对称地甲基化MICU1,导致Ca(2+)敏感性降低。UCP2 / 3标准化甲基化的MICU1的Ca(2+)敏感性,并因此重新建立线粒体Ca(2+)的吸收活性。这些数据提供了由PRMT1和UCP2 / 3对线粒体Ca(2+)单向转运体的复杂调控的新颖见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号