当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibria of Aqueous Ternary Systems Li2SO4 + Na2SO4 + H2O and Na2SO4 + K2SO4 + H2O at 303.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-01-06 , DOI: 10.1021/acs.jced.2c00673
Xudong Yu 1 , Zhihao Yao 1 , Zhixing Zhao 1 , Li Du 1 , Xia Feng 1 , Siying Ren 1 , Jun Luo 1 , Ying Zeng 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-01-06 , DOI: 10.1021/acs.jced.2c00673
Xudong Yu 1 , Zhihao Yao 1 , Zhixing Zhao 1 , Li Du 1 , Xia Feng 1 , Siying Ren 1 , Jun Luo 1 , Ying Zeng 1
Affiliation
|
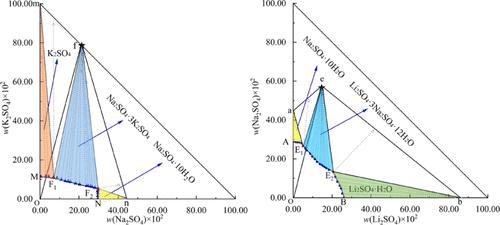
The phase equilibria of ternary systems Li2SO4 + Na2SO4 + H2O and Na2SO4 + K2SO4 + H2O at 303.2 K were investigated by using the isothermal dissolution method. The solubility, density, and refractive index of the systems were measured. There are two ternary invariant points and three crystallization regions corresponding to Li2SO4·3Na2SO4·12H2O, Li2SO4·H2O, and Na2SO4·10H2O in the system Li2SO4 + Na2SO4 + H2O at 303.2 K. There are two ternary invariant points and three crystallization regions corresponding to Na2SO4·10H2O, Na2SO4·3K2SO4, and K2SO4 in the system Na2SO4 + K2SO4 + H2O at 303.2 K. By comparing the phase diagrams of the ternary system Li2SO4 + Na2SO4 + H2O at different temperatures, one can find that the existence form of double salt changes from Li2SO4·3Na2SO4·12H2O to Li2SO4·Na2SO4. By comparing the phase diagrams of the ternary system Na2SO4 + K2SO4 + H2O at different temperatures, the double salt Na2SO4·3K2SO4 was not found at 273.2 K, while it was formed at 303.2 and 313.2 K. Meanwhile, the thermodynamic data of these two systems at 303.2 K were fitted by the Pitzer–Simonson–Clegg model, and the calculated values agree well with the experimental values.
中文翻译:

水系三元体系 Li2SO4 + Na2SO4 + H2O 和 Na2SO4 + K2SO4 + H2O 在 303.2 K 下的相平衡
采用等温溶解法研究了三元体系Li 2 SO 4 + Na 2 SO 4 + H 2 O和Na 2 SO 4 + K 2 SO 4 + H 2 O在303.2 K时的相平衡。测量系统的溶解度、密度和折射率。Li 2 SO 4 ·3Na 2 SO 4 ·12H 2 O、Li 2 SO 4 ·H 2 O、Na分别对应两个三元不变点和三个结晶区2 SO 4 ·10H 2 O体系中Li 2 SO 4 + Na 2 SO 4 + H 2 O在303.2 K时有两个三元不变点和三个结晶区分别对应Na 2 SO 4 ·10H 2 O、Na 2 SO 4 ·3K 2 SO 4和K 2 SO 4体系中Na 2 SO 4 + K 2 SO 4 + H 2O at 303.2 K。对比三元体系Li 2 SO 4 + Na 2 SO 4 + H 2 O在不同温度下的相图,可以发现复盐的存在形式由Li 2 SO 4 ·3Na 2 SO 4 ·12H 2 O为Li 2 SO 4 ·Na 2 SO 4。通过比较三元体系Na 2 SO 4 + K 2 SO 4 + H 2 O在不同温度下的相图,复盐Na2 SO 4 ·3K 2 SO 4在273.2 K时未发现,而在303.2和313.2 K时形成。同时,这两个体系在303.2 K时的热力学数据采用Pitzer-Simonson-Clegg模型进行拟合,得到计算值与实验值吻合良好。
更新日期:2023-01-06
中文翻译:

水系三元体系 Li2SO4 + Na2SO4 + H2O 和 Na2SO4 + K2SO4 + H2O 在 303.2 K 下的相平衡
采用等温溶解法研究了三元体系Li 2 SO 4 + Na 2 SO 4 + H 2 O和Na 2 SO 4 + K 2 SO 4 + H 2 O在303.2 K时的相平衡。测量系统的溶解度、密度和折射率。Li 2 SO 4 ·3Na 2 SO 4 ·12H 2 O、Li 2 SO 4 ·H 2 O、Na分别对应两个三元不变点和三个结晶区2 SO 4 ·10H 2 O体系中Li 2 SO 4 + Na 2 SO 4 + H 2 O在303.2 K时有两个三元不变点和三个结晶区分别对应Na 2 SO 4 ·10H 2 O、Na 2 SO 4 ·3K 2 SO 4和K 2 SO 4体系中Na 2 SO 4 + K 2 SO 4 + H 2O at 303.2 K。对比三元体系Li 2 SO 4 + Na 2 SO 4 + H 2 O在不同温度下的相图,可以发现复盐的存在形式由Li 2 SO 4 ·3Na 2 SO 4 ·12H 2 O为Li 2 SO 4 ·Na 2 SO 4。通过比较三元体系Na 2 SO 4 + K 2 SO 4 + H 2 O在不同温度下的相图,复盐Na2 SO 4 ·3K 2 SO 4在273.2 K时未发现,而在303.2和313.2 K时形成。同时,这两个体系在303.2 K时的热力学数据采用Pitzer-Simonson-Clegg模型进行拟合,得到计算值与实验值吻合良好。

































 京公网安备 11010802027423号
京公网安备 11010802027423号