Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrin expression and extracellular matrix adhesion of septoclasts, pericytes, and endothelial cells at the chondro-osseous junction and the metaphysis of the proximal tibia in young mice
Journal of Anatomy ( IF 1.8 ) Pub Date : 2023-01-05 , DOI: 10.1111/joa.13820 Yasuhiko Bando 1 , Arata Nagasaka 1 , Go Onozawa 1, 2 , Koji Sakiyama 3 , Yuji Owada 4 , Osamu Amano 1
Journal of Anatomy ( IF 1.8 ) Pub Date : 2023-01-05 , DOI: 10.1111/joa.13820 Yasuhiko Bando 1 , Arata Nagasaka 1 , Go Onozawa 1, 2 , Koji Sakiyama 3 , Yuji Owada 4 , Osamu Amano 1
Affiliation
|
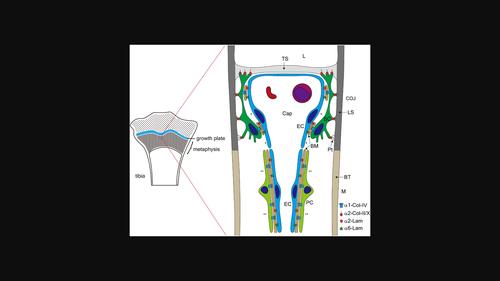
We previously reported that septoclasts, which are uncalcified growth plate (GP) cartilage matrix-resorbing cells, are derived from pericytes surrounding capillary endothelial cells. Resorption of the GP is assumed to be regulated synchronously by septoclasts, pericytes, and endothelial cells. To reveal the contribution of the extracellular matrix (ECM) to the regulatory mechanisms of septoclastic cartilage resorption, we investigated the spatial correlation between the cells and the ECM in the GP matrix and basement membrane (BM) and investigated the expression of integrins—ECM receptors—in the cells. Septoclasts attached to the transverse septa containing collagen-II/-X at the tip of their processes and to the longitudinal septa containing collagen-II/-X at the spine-like processes extending from their bodies and processes. Collagen-IV and laminin α4 in the BM were sparsely detected between septoclasts and capillary endothelial cells at the chondro-osseous junction (COJ) and were absent in the outer surface of pericytes at the metaphysis. Integrin α1/α2, integrin α1, and integrin α2/α6 were detected in the cell membranes of septoclasts, pericytes, and endothelial cells, respectively. These results suggest that the adhesion between septoclasts and the cartilage ECM forming the scaffolds for cartilage resorption and migration is provided by integrin α2–collagen-II/-X interaction and that the adhesions between the BM and pericytes or endothelial cells are mediated by integrin α1–collagen-IV and integrin α2/α6–laminin interaction, respectively.
中文翻译:

幼鼠软骨交界处隔膜细胞、周细胞和内皮细胞以及胫骨近端干骺端的整合素表达和细胞外基质粘附
我们之前报道过,隔膜破细胞是未钙化的生长板 (GP) 软骨基质吸收细胞,来源于毛细血管内皮细胞周围的周细胞。假设 GP 的重吸收受隔膜细胞、周细胞和内皮细胞的同步调节。为了揭示细胞外基质 (ECM) 对间隔碎屑软骨吸收调节机制的贡献,我们研究了细胞与 GP 基质和基底膜 (BM) 中 ECM 之间的空间相关性,并研究了细胞中整合素 - ECM 受体 - 的表达。隔膜细胞附着在横隔膜上,在其突起的尖端含有胶原蛋白-II/-X,在从其身体和过程中延伸出来的脊柱状突起附着在含有胶原蛋白-II/-X 的纵向隔膜上。BM 中的胶原 IV 和层粘连蛋白 α4 在软骨-骨交界处 (COJ) 的隔膜细胞和毛细血管内皮细胞之间稀疏检测到,而在干骺端的周细胞外表面不存在。分别在隔膜细胞、周细胞和内皮细胞的细胞膜中检测到整合素 α1/α2 、整合素 α1 和整合素 α2/α6。这些结果表明,隔膜破细胞与形成软骨吸收和迁移支架的软骨 ECM 之间的粘附是由整合素 α2-胶原蛋白-II/-X 相互作用提供的,而 BM 与周细胞或内皮细胞之间的粘附分别由整合素 α1-胶原蛋白-IV 和整合素 α2/α6-层粘连蛋白相互作用介导。
更新日期:2023-01-05
中文翻译:

幼鼠软骨交界处隔膜细胞、周细胞和内皮细胞以及胫骨近端干骺端的整合素表达和细胞外基质粘附
我们之前报道过,隔膜破细胞是未钙化的生长板 (GP) 软骨基质吸收细胞,来源于毛细血管内皮细胞周围的周细胞。假设 GP 的重吸收受隔膜细胞、周细胞和内皮细胞的同步调节。为了揭示细胞外基质 (ECM) 对间隔碎屑软骨吸收调节机制的贡献,我们研究了细胞与 GP 基质和基底膜 (BM) 中 ECM 之间的空间相关性,并研究了细胞中整合素 - ECM 受体 - 的表达。隔膜细胞附着在横隔膜上,在其突起的尖端含有胶原蛋白-II/-X,在从其身体和过程中延伸出来的脊柱状突起附着在含有胶原蛋白-II/-X 的纵向隔膜上。BM 中的胶原 IV 和层粘连蛋白 α4 在软骨-骨交界处 (COJ) 的隔膜细胞和毛细血管内皮细胞之间稀疏检测到,而在干骺端的周细胞外表面不存在。分别在隔膜细胞、周细胞和内皮细胞的细胞膜中检测到整合素 α1/α2 、整合素 α1 和整合素 α2/α6。这些结果表明,隔膜破细胞与形成软骨吸收和迁移支架的软骨 ECM 之间的粘附是由整合素 α2-胶原蛋白-II/-X 相互作用提供的,而 BM 与周细胞或内皮细胞之间的粘附分别由整合素 α1-胶原蛋白-IV 和整合素 α2/α6-层粘连蛋白相互作用介导。































 京公网安备 11010802027423号
京公网安备 11010802027423号