当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-heterocyclic carbene-catalyzed direct enantioselective synthesis of dihydroisoxazolo[5,4-b]pyridin-6-ones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-01-06 , DOI: 10.1039/d2qo01736a Xiaoxia Huang 1 , Yarui Li 1 , Jieyin He 1 , Shiyong Peng 1 , Jian Wang 1 , Ming Lang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-01-06 , DOI: 10.1039/d2qo01736a Xiaoxia Huang 1 , Yarui Li 1 , Jieyin He 1 , Shiyong Peng 1 , Jian Wang 1 , Ming Lang 1
Affiliation
|
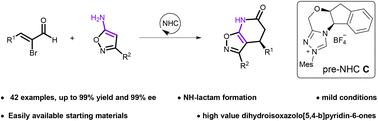
A novel N-heterocyclic carbene (NHC)-catalyzed asymmetric [3 + 3] cycloaddition between α-bromoenals and 5-aminoisoxazoles has been developed. With this method, various dihydroisoxazolo[5,4-b]pyridin-6-ones were obtained in high yields (up to 99%) with excellent enantioselectivities (up to >99%) under mild conditions. Notably, this is the first example of direct catalytic asymmetric synthesis of dihydroisoxazolo[5,4-b]pyridin-6-ones.
中文翻译:

N-杂环卡宾催化直接对映选择性合成二氢异恶唑并[5,4-b]吡啶-6-酮
开发了一种新型 N-杂环卡宾 (NHC) 催化的 α-溴烯醛和 5-氨基异恶唑之间的不对称 [3 + 3] 环加成反应。利用该方法,在温和条件下以高产率(高达 99%)和优异的对映选择性(高达 >99%)获得了各种二氢异恶唑并[5,4 - b ]吡啶-6-酮。值得注意的是,这是直接催化不对称合成二氢异恶唑并[5,4 - b ]吡啶-6-酮的第一个例子。
更新日期:2023-01-06
中文翻译:

N-杂环卡宾催化直接对映选择性合成二氢异恶唑并[5,4-b]吡啶-6-酮
开发了一种新型 N-杂环卡宾 (NHC) 催化的 α-溴烯醛和 5-氨基异恶唑之间的不对称 [3 + 3] 环加成反应。利用该方法,在温和条件下以高产率(高达 99%)和优异的对映选择性(高达 >99%)获得了各种二氢异恶唑并[5,4 - b ]吡啶-6-酮。值得注意的是,这是直接催化不对称合成二氢异恶唑并[5,4 - b ]吡啶-6-酮的第一个例子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号