Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mace-Like Plasmonic Au-Pd Heterostructures Boost Near-Infrared Photoimmunotherapy
Advanced Science ( IF 14.3 ) Pub Date : 2023-01-04 , DOI: 10.1002/advs.202204842 Yanlin Feng 1 , Xin Ning 1 , Jianlin Wang 1 , Zhaoyang Wen 1 , Fangfang Cao 2, 3 , Qing You 2, 3 , Jianhua Zou 2, 3 , Xin Zhou 1 , Teng Sun 1 , Jimin Cao 1 , Xiaoyuan Chen 2, 3, 4, 5
Advanced Science ( IF 14.3 ) Pub Date : 2023-01-04 , DOI: 10.1002/advs.202204842 Yanlin Feng 1 , Xin Ning 1 , Jianlin Wang 1 , Zhaoyang Wen 1 , Fangfang Cao 2, 3 , Qing You 2, 3 , Jianhua Zou 2, 3 , Xin Zhou 1 , Teng Sun 1 , Jimin Cao 1 , Xiaoyuan Chen 2, 3, 4, 5
Affiliation
|
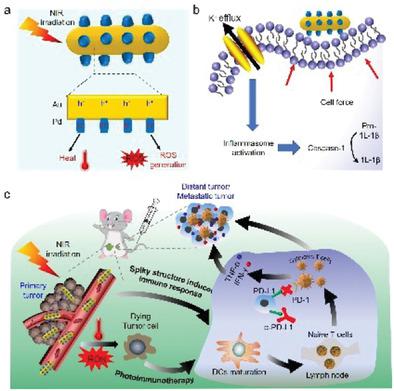
Photoimmunotherapy, with spatiotemporal precision and noninvasive property, has provided a novel targeted therapeutic strategy for highly malignant triple-negative breast cancer (TNBC). However, their therapeutic effect is severely restricted by the insufficient generation of tumor antigens and the weak activation of immune response, which is caused by the limited tissue penetration of light and complex immunosuppressive microenvironment. To improve the outcomes, herein, mace-like plasmonic AuPd heterostructures (Au Pd HSs) have been fabricated to boost near-infrared (NIR) photoimmunotherapy. The plasmonic Au Pd HSs exhibit strong photothermal and photodynamic effects under NIR light irradiation, effectively triggering immunogenic cell death (ICD) to activate the immune response. Meanwhile, the spiky surface of Au Pd HSs can also stimulate the maturation of DCs to present these antigens, amplifying the immune response. Ultimately, combining with anti-programmed death-ligand 1 (α-PD-L1) will further reverse the immunosuppressive microenvironment and enhance the infiltration of cytotoxic T lymphocytes (CTLs), not only eradicating primary TNBC but also completely inhibiting mimetic metastatic TNBC. Overall, the current study opens a new path for the treatment of TNBC through immunotherapy by integrating nanotopology and plasmonic performance.
中文翻译:

类狼牙棒等离激元金钯异质结构促进近红外光免疫疗法
光免疫疗法具有时空精确性和无创性,为高度恶性三阴性乳腺癌(TNBC)提供了一种新颖的靶向治疗策略。然而,由于光和复杂的免疫抑制微环境的组织穿透性有限,导致肿瘤抗原生成不足和免疫反应激活弱,其治疗效果受到严重限制。为了改善结果,本文中制造了类似狼牙棒的等离激元金-钯异质结构(Au Pd HS)来增强近红外(NIR)光免疫疗法。等离子体Au Pd HS在近红外光照射下表现出强烈的光热和光动力效应,有效触发免疫原性细胞死亡(ICD)以激活免疫反应。同时,Au Pd HS 的尖刺表面还可以刺激 DC 成熟以呈递这些抗原,从而放大免疫反应。最终,与抗程序性死亡配体1( α -PD-L1)结合将进一步逆转免疫抑制微环境,增强细胞毒性T淋巴细胞(CTL)的浸润,不仅根除原发性TNBC,而且完全抑制模拟转移性TNBC。总体而言,当前的研究通过整合纳米拓扑和等离子体性能,为通过免疫疗法治疗 TNBC 开辟了一条新途径。
更新日期:2023-01-04
中文翻译:

类狼牙棒等离激元金钯异质结构促进近红外光免疫疗法
光免疫疗法具有时空精确性和无创性,为高度恶性三阴性乳腺癌(TNBC)提供了一种新颖的靶向治疗策略。然而,由于光和复杂的免疫抑制微环境的组织穿透性有限,导致肿瘤抗原生成不足和免疫反应激活弱,其治疗效果受到严重限制。为了改善结果,本文中制造了类似狼牙棒的等离激元金-钯异质结构(Au Pd HS)来增强近红外(NIR)光免疫疗法。等离子体Au Pd HS在近红外光照射下表现出强烈的光热和光动力效应,有效触发免疫原性细胞死亡(ICD)以激活免疫反应。同时,Au Pd HS 的尖刺表面还可以刺激 DC 成熟以呈递这些抗原,从而放大免疫反应。最终,与抗程序性死亡配体1( α -PD-L1)结合将进一步逆转免疫抑制微环境,增强细胞毒性T淋巴细胞(CTL)的浸润,不仅根除原发性TNBC,而且完全抑制模拟转移性TNBC。总体而言,当前的研究通过整合纳米拓扑和等离子体性能,为通过免疫疗法治疗 TNBC 开辟了一条新途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号