当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A strategic approach for Csp3–H functionalization of 9H-fluorene: an acceptorless dehydrogenation and borrowing hydrogen approach
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2023-01-05 , DOI: 10.1039/d2cy02060b Rahul Sharma 1 , Avijit Mondal 1 , Arup Samanta 1 , Dipankar Srimani 1
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2023-01-05 , DOI: 10.1039/d2cy02060b Rahul Sharma 1 , Avijit Mondal 1 , Arup Samanta 1 , Dipankar Srimani 1
Affiliation
|
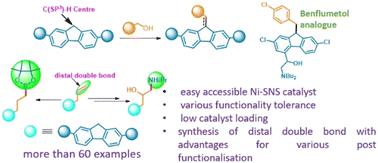
Herein, we described the selective synthesis of both alkylated and alkenylated fluorenes using a single SNS ligand derived nickel complex. The protocol was employed for a wide range of substrates, including substituted fluorenes and various alcohols including aliphatic alcohols with a distal double bond. To expand the synthetic utility, an antimalarial drug analogue, benflumetol, was synthesized, and various post-modifications of alkylated fluorenes to their corresponding epoxides, amino alcohols, and boronate esters were demonstrated. Control experiments and kinetic profile diagrams depict that the reaction works via an unsaturated intermediate and proves the involvement of the borrowing hydrogen approach.
中文翻译:

9H-芴的 Csp3–H 功能化的战略方法:无受体脱氢和借氢方法
在此,我们描述了使用单一 SNS 配体衍生的镍络合物选择性合成烷基化和烯基化芴。该协议适用于范围广泛的底物,包括取代的芴和各种醇,包括具有远端双键的脂肪醇。为了扩大合成效用,合成了一种抗疟药类似物苯氟美醇,并证明了烷基化芴对其相应的环氧化物、氨基醇和硼酸酯的各种后修饰。控制实验和动力学曲线图描述了该反应通过不饱和中间体起作用,并证明了借氢方法的参与。
更新日期:2023-01-05
中文翻译:

9H-芴的 Csp3–H 功能化的战略方法:无受体脱氢和借氢方法
在此,我们描述了使用单一 SNS 配体衍生的镍络合物选择性合成烷基化和烯基化芴。该协议适用于范围广泛的底物,包括取代的芴和各种醇,包括具有远端双键的脂肪醇。为了扩大合成效用,合成了一种抗疟药类似物苯氟美醇,并证明了烷基化芴对其相应的环氧化物、氨基醇和硼酸酯的各种后修饰。控制实验和动力学曲线图描述了该反应通过不饱和中间体起作用,并证明了借氢方法的参与。















































 京公网安备 11010802027423号
京公网安备 11010802027423号