当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
[1,2,4]Triazolo[3,4-b]benzothiazole Scaffold as Versatile Nicotinamide Mimic Allowing Nanomolar Inhibition of Different PARP Enzymes
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-01-04 , DOI: 10.1021/acs.jmedchem.2c01460 Sudarshan Murthy 1 , Maria Giulia Nizi 2 , Mirko M Maksimainen 1 , Serena Massari 2 , Juho Alaviuhkola 1 , Barbara E Lippok 3 , Chiara Vagaggini 4 , Sven T Sowa 1 , Albert Galera-Prat 1 , Yashwanth Ashok 1 , Harikanth Venkannagari 1 , Renata Prunskaite-Hyyryläinen 1 , Elena Dreassi 4 , Bernhard Lüscher 3 , Patricia Korn 3 , Oriana Tabarrini 2 , Lari Lehtiö 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-01-04 , DOI: 10.1021/acs.jmedchem.2c01460 Sudarshan Murthy 1 , Maria Giulia Nizi 2 , Mirko M Maksimainen 1 , Serena Massari 2 , Juho Alaviuhkola 1 , Barbara E Lippok 3 , Chiara Vagaggini 4 , Sven T Sowa 1 , Albert Galera-Prat 1 , Yashwanth Ashok 1 , Harikanth Venkannagari 1 , Renata Prunskaite-Hyyryläinen 1 , Elena Dreassi 4 , Bernhard Lüscher 3 , Patricia Korn 3 , Oriana Tabarrini 2 , Lari Lehtiö 1
Affiliation
|
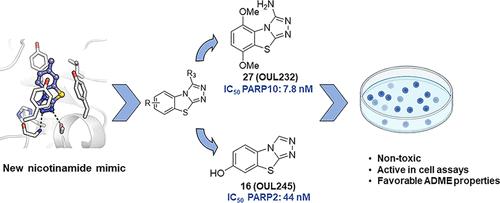
We report [1,2,4]triazolo[3,4-b]benzothiazole (TBT) as a new inhibitor scaffold, which competes with nicotinamide in the binding pocket of human poly- and mono-ADP-ribosylating enzymes. The binding mode was studied through analogues and cocrystal structures with TNKS2, PARP2, PARP14, and PARP15. Based on the substitution pattern, we were able to identify 3-amino derivatives 21 (OUL243) and 27 (OUL232) as inhibitors of mono-ARTs PARP7, PARP10, PARP11, PARP12, PARP14, and PARP15 at nM potencies, with 27 being the most potent PARP10 inhibitor described to date (IC50 of 7.8 nM) and the first PARP12 inhibitor ever reported. On the contrary, hydroxy derivative 16 (OUL245) inhibits poly-ARTs with a selectivity toward PARP2. The scaffold does not possess inherent cell toxicity, and the inhibitors can enter cells and engage with the target protein. This, together with favorable ADME properties, demonstrates the potential of TBT scaffold for future drug development efforts toward selective inhibitors against specific enzymes.
中文翻译:

[1,2,4]三唑并 [3,4-b] 苯并噻唑支架作为多功能烟酰胺模拟物允许不同 PARP 酶的纳摩尔抑制
我们将 [1,2,4] 三唑并 [3,4- b ] 苯并噻唑 (TBT) 报告为一种新的抑制剂支架,它与烟酰胺在人类聚-和单-ADP-核糖基化酶的结合口袋中竞争。通过 TNKS2、PARP2、PARP14 和 PARP15 的类似物和共晶结构研究了结合模式。基于取代模式,我们能够将 3-氨基衍生物21 (OUL243) 和27 (OUL232) 鉴定为 nM 效价的单 ART PARP7、PARP10、PARP11、PARP12、PARP14 和 PARP15 抑制剂,其中27是迄今为止描述的最有效的 PARP10 抑制剂(IC 50为 7.8 nM)和第一个报道的 PARP12 抑制剂。相反,羟基衍生物16(OUL245) 抑制对 PARP2 具有选择性的多 ART。支架不具有固有的细胞毒性,抑制剂可以进入细胞并与靶蛋白结合。这与有利的 ADME 特性一起,证明了 TBT 支架在未来针对特定酶的选择性抑制剂的药物开发工作中的潜力。
更新日期:2023-01-04
中文翻译:

[1,2,4]三唑并 [3,4-b] 苯并噻唑支架作为多功能烟酰胺模拟物允许不同 PARP 酶的纳摩尔抑制
我们将 [1,2,4] 三唑并 [3,4- b ] 苯并噻唑 (TBT) 报告为一种新的抑制剂支架,它与烟酰胺在人类聚-和单-ADP-核糖基化酶的结合口袋中竞争。通过 TNKS2、PARP2、PARP14 和 PARP15 的类似物和共晶结构研究了结合模式。基于取代模式,我们能够将 3-氨基衍生物21 (OUL243) 和27 (OUL232) 鉴定为 nM 效价的单 ART PARP7、PARP10、PARP11、PARP12、PARP14 和 PARP15 抑制剂,其中27是迄今为止描述的最有效的 PARP10 抑制剂(IC 50为 7.8 nM)和第一个报道的 PARP12 抑制剂。相反,羟基衍生物16(OUL245) 抑制对 PARP2 具有选择性的多 ART。支架不具有固有的细胞毒性,抑制剂可以进入细胞并与靶蛋白结合。这与有利的 ADME 特性一起,证明了 TBT 支架在未来针对特定酶的选择性抑制剂的药物开发工作中的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号