Synlett ( IF 1.7 ) Pub Date : 2023-01-03 , DOI: 10.1055/a-1992-6757 Hao Liang 1 , Jun Wang 1
|
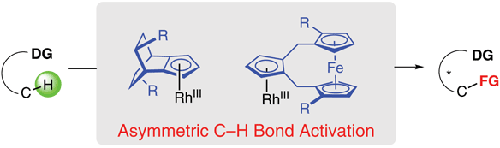
To fulfill increasing and diverse demands of cyclopentadienyl metal complexes (CpM) catalyzed asymmetric C–H activation, innovation in developing new types of chiral Cp ligands should be important. In this context, we found the structurally rigid chiral bicyclo[2.2.2]octane-fused Cp ligands originated by Vollhardt and Halterman were efficient to enable highly enantioselective C–H activation of N-methoxybenzamides with quinones. Besides, we also designed and synthesized a class of ferrocene-based chiral Cp ligand featuring a loose chiral pocket, whose rhodium complexes exhibited high reactivity and reasonable enantioselectivity in an asymmetric intramolecular aryl amination of alkene.
1 Introduction
2 Chiral Cp Ligands Applied in Asymmetric C–H Activation
3 Chiral Bicyclo[2.2.2]octane-Fused Cp Ligands
4 Chiral Ferrocene-Derived Cp Ligands
5 Conclusion
中文翻译:

用于不对称 C-H 活化的手性双环 [2.2.2] 辛烷稠合和二茂铁衍生的环戊二烯基配体
为了满足对环戊二烯基金属配合物 (CpM) 催化不对称 C-H 活化日益增长和多样化的需求,开发新型手性 Cp 配体的创新应该很重要。在这种情况下,我们发现由 Vollhardt 和 Halterman 发明的结构刚性手性双环[2.2.2] 辛烷稠合 Cp 配体可有效实现N-甲氧基苯甲酰胺与醌类的高度对映选择性 C-H 活化。此外,我们还设计合成了一类具有松散手性口袋的二茂铁基手性Cp配体,其铑配合物在烯烃的不对称分子内芳基胺化反应中表现出高反应活性和合理的对映选择性。
1 简介
2 应用于不对称C-H活化的手性Cp配体
3 手性双环[2.2.2]辛烷稠合Cp配体
4 手性二茂铁衍生的 Cp 配体
5 结论































 京公网安备 11010802027423号
京公网安备 11010802027423号