Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design and synthesis of a clickable, photoreactive amino acid p-(4-(but-3-yn-1-yl)benzoyl)-L-phenylalanine for peptide photoaffinity labeling
RSC Advances ( IF 3.9 ) Pub Date : 2023-01-04 , DOI: 10.1039/d2ra07248c Penggang Han 1 , Fuli Wang 1 , Shaoheng Bao 1 , Ge Yao 1 , Xiukun Wan 1 , JiaJia Liu 1 , Hui Jiang 1
RSC Advances ( IF 3.9 ) Pub Date : 2023-01-04 , DOI: 10.1039/d2ra07248c Penggang Han 1 , Fuli Wang 1 , Shaoheng Bao 1 , Ge Yao 1 , Xiukun Wan 1 , JiaJia Liu 1 , Hui Jiang 1
Affiliation
|
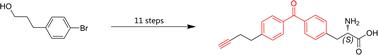
Photoaffinity labeling is a powerful technique to investigate the interactions between bioactive peptides and their targets. To construct a peptide-derived photoaffinity probe, at least two amino acids need to be modified or replaced, increasing experimental difficulties and negatively affecting activity. Herein, we report the synthesis of a clickable, photoreactive amino acid p-(4-(but-3-yn-1-yl)benzoyl)-L-phenylalanine (Abpa) and its Fmoc-protected version from 3-(4-bromophenyl)-1-propanol in 11 steps with an overall 12.5% yield. The amino acid contains both a photoreactive benzophenone and a clickable terminal alkyne which acts like a reporter tag by fast attachment to other functional groups via ‘click’ reaction, and a photoaffinity probe could be created by one single amino acid substitution during peptide synthesis. And its small size helps to retain bioactivity. The efficiency of Abpa was demonstrated by photoaffinity labeling experiments using photoactivatable probes of α-conotoxin MI.
中文翻译:

用于肽光亲和标记的可点击光反应性氨基酸 p-(4-(but-3-yn-1-yl)benzoyl)-L-phenylalanine 的设计与合成
光亲和标记是研究生物活性肽与其靶标之间相互作用的强大技术。要构建肽衍生的光亲和探针,至少需要修改或替换两个氨基酸,增加了实验难度并对活性产生负面影响。在此,我们报告了一种可点击的光反应性氨基酸p -(4-(but-3-yn-1-yl)benzoyl)- L -phenylalanine (Abpa) 及其 Fmoc 保护版本的合成,来自 3-(4-溴苯基)-1-丙醇分11步合成,总收率为12.5%。该氨基酸包含光反应性二苯甲酮和可点击的末端炔烃,后者通过快速连接到其他官能团来充当报告标签“点击”反应,并且可以在肽合成过程中通过一个单一的氨基酸取代来产生光亲和探针。而且它的小尺寸有助于保持生物活性。使用 α-芋螺毒素 MI 的光敏探针通过光亲和标记实验证明了 Abpa 的效率。
更新日期:2023-01-04
中文翻译:

用于肽光亲和标记的可点击光反应性氨基酸 p-(4-(but-3-yn-1-yl)benzoyl)-L-phenylalanine 的设计与合成
光亲和标记是研究生物活性肽与其靶标之间相互作用的强大技术。要构建肽衍生的光亲和探针,至少需要修改或替换两个氨基酸,增加了实验难度并对活性产生负面影响。在此,我们报告了一种可点击的光反应性氨基酸p -(4-(but-3-yn-1-yl)benzoyl)- L -phenylalanine (Abpa) 及其 Fmoc 保护版本的合成,来自 3-(4-溴苯基)-1-丙醇分11步合成,总收率为12.5%。该氨基酸包含光反应性二苯甲酮和可点击的末端炔烃,后者通过快速连接到其他官能团来充当报告标签“点击”反应,并且可以在肽合成过程中通过一个单一的氨基酸取代来产生光亲和探针。而且它的小尺寸有助于保持生物活性。使用 α-芋螺毒素 MI 的光敏探针通过光亲和标记实验证明了 Abpa 的效率。








































 京公网安备 11010802027423号
京公网安备 11010802027423号