当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aryne Atropisomers: Chiral Arynes for the Enantiospecific Synthesis of Atropisomers and Nanographene Atropisomers
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-01-03 , DOI: 10.1021/acs.accounts.2c00575 Yoann Coquerel 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-01-03 , DOI: 10.1021/acs.accounts.2c00575 Yoann Coquerel 1
Affiliation
|
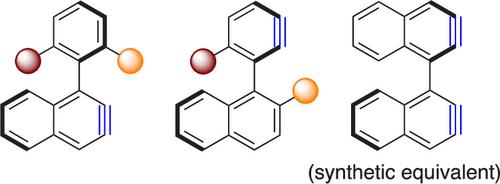
The basics about arynes and their applications in synthetic organic chemistry are briefly presented, and the concept of atropisomerism is defined, highlighting that it is a time-dependent form of isomerism and chirality. It is remembered that racemization is a macroscopic and statistical irreversible process, while enantiomerization is a nanoscopic reversible process that occurs at the molecular scale, with racemization being twice as fast as enantiomerization. The concept of aryne atropisomers is introduced with a naive question: Can synthetically useful nonracemic aryne atropisomers having a triple bond ortho to the stereogenic single bond exist in solution? It was found that such aryne atropisomers can be generated in solution from easily available ortho-iodoaryl triflate precursors and excess trimethylsilylmethylmagnesium chloride. Analysis of the barriers to enantiomerization of some aryne atropisomers by computational modeling revealed the key contribution to the configurational stability of the H atom in tris-ortho-substituted biphenyl-based atropisomers. Using a specially designed prototype of aryne atropisomer, for which the barrier to enantiomerization was accurately evaluated by advanced computational modeling, the kinetic parameters of its reaction with furan were experimentally determined. From these measurements, it was concluded that any aryne atropisomer with a barrier to enantiomerization ΔGenant⧧ equal to or higher than 50 kJ mol–1 would lead to fully enantiospecific reactions. The synthetic applications of two structurally distinct aryne atropisomers built on a 1-phenylnaphthalene platform are described: one has the aryne triple bond embedded in the naphthyl moiety, and the other has the aryne triple bond embedded in the phenyl moiety. Both aryne atropisomers allowed for the fully enantiospecific, and possibly overall enantioselective, syntheses of original atropisomers based on standard aryne chemistry. For instance, reactions with anthracene and perylene afforded triptycene and nanographene atropisomers, respectively, in high enantiomeric excesses. A bis(aryne) atropisomer synthetic equivalent prepared from either enantiomer of BINOL is described for 3D bidirectional reactions with a single handedness. Its 2-fold reactions with anthracene and perylene afforded the corresponding severely congested bis(benzotriptycene) (99% ee) nanocarbon atropisomer and bis(anthra[1,2,3,4-ghi]perylene) (98% ee) nanographene atropisomer, respectively. This allowed the discovery of bis(twistacene) atropisomers as a new class of polycyclic aromatic hydrocarbons (PAH) with multiple stereogenicities. Cross reactions with the bis(aryne) atropisomer synthetic equivalent and two different arynophiles proved feasible, providing a nanographene atropisomer with a benzotriptycene unit and an anthra[1,2,3,4-ghi]perylene unit assembled around a stereogenic axis as a unique chiral PAH (99% ee). Overall, because the concept is simple and its implementation is easy, aryne atropisomers is an attractive approach to the synthesis of atropisomers in a broad meaning. Applications to the synthesis of large PAH atropisomers with single handedness are particularly promising.
中文翻译:

芳炔阻转异构体:手性芳炔用于阻转异构体和纳米石墨烯阻转异构体的对映特异性合成
简要介绍了芳烃的基础知识及其在有机合成化学中的应用,定义了阻转异构的概念,强调它是一种随时间变化的异构和手性形式。请记住,外消旋化是一个宏观的统计不可逆过程,而对映异构化是发生在分子尺度的纳米级可逆过程,外消旋化的速度是对映异构化的两倍。芳炔阻转异构体的概念是通过一个天真的问题引入的:具有与立构单键邻位的三键的合成有用的非外消旋芳炔阻转异构体能否存在于溶液中?人们发现,这种芳炔阻转异构体可以在溶液中从容易获得的原位生成-碘代芳基三氟甲磺酸酯前体和过量的三甲基甲硅烷基甲基氯化镁。通过计算模型分析某些芳炔阻转异构体对映异构化的障碍,揭示了对三邻位取代联苯基阻转异构体中 H 原子构型稳定性的关键贡献。使用专门设计的芳烃阻转异构体原型,通过先进的计算模型准确评估了对映异构化的障碍,通过实验确定了其与呋喃反应的动力学参数。从这些测量中可以得出结论,任何具有对映异构化势垒的芳炔阻转异构体 Δ G对映体⧧等于或高于 50 kJ mol –1会导致完全对映特异性反应。描述了在 1-苯基萘平台上构建的两种结构不同的芳炔阻转异构体的合成应用:一种芳炔三键嵌入萘基部分,另一种芳炔三键嵌入苯基部分。两种芳炔阻转异构体都允许基于标准芳炔化学的完全对映特异性和可能的整体对映选择性合成原始阻转异构体。例如,与蒽和苝的反应分别以高对映体过量提供三蝶烯和纳米石墨烯阻转异构体。描述了由 BINOL 的任一对映异构体制备的双(芳烃)阻转异构体合成等效物,用于具有单手性的 3D 双向反应。ghi ]perylene) (98% ee) 纳米石墨烯阻转异构体。这允许发现双(twistacene)阻转异构体作为一类具有多重立体异构性的新型多环芳烃(PAH)。与双(芳烃)阻转异构体合成等价物和两种不同的亲吻化合物的交叉反应被证明是可行的,提供了一种具有苯并三蝶烯单元和蒽[1,2,3,4- ghi ]苝单元的纳米石墨烯阻转异构体,该单元围绕立体异构轴组装,作为独特的手性 PAH (99% ee)。总的来说,由于概念简单且易于实施,芳炔阻转异构体是广义上阻转异构体合成的一种有吸引力的方法。应用于合成具有单手性的大型 PAH 阻转异构体特别有前途。
更新日期:2023-01-03
中文翻译:

芳炔阻转异构体:手性芳炔用于阻转异构体和纳米石墨烯阻转异构体的对映特异性合成
简要介绍了芳烃的基础知识及其在有机合成化学中的应用,定义了阻转异构的概念,强调它是一种随时间变化的异构和手性形式。请记住,外消旋化是一个宏观的统计不可逆过程,而对映异构化是发生在分子尺度的纳米级可逆过程,外消旋化的速度是对映异构化的两倍。芳炔阻转异构体的概念是通过一个天真的问题引入的:具有与立构单键邻位的三键的合成有用的非外消旋芳炔阻转异构体能否存在于溶液中?人们发现,这种芳炔阻转异构体可以在溶液中从容易获得的原位生成-碘代芳基三氟甲磺酸酯前体和过量的三甲基甲硅烷基甲基氯化镁。通过计算模型分析某些芳炔阻转异构体对映异构化的障碍,揭示了对三邻位取代联苯基阻转异构体中 H 原子构型稳定性的关键贡献。使用专门设计的芳烃阻转异构体原型,通过先进的计算模型准确评估了对映异构化的障碍,通过实验确定了其与呋喃反应的动力学参数。从这些测量中可以得出结论,任何具有对映异构化势垒的芳炔阻转异构体 Δ G对映体⧧等于或高于 50 kJ mol –1会导致完全对映特异性反应。描述了在 1-苯基萘平台上构建的两种结构不同的芳炔阻转异构体的合成应用:一种芳炔三键嵌入萘基部分,另一种芳炔三键嵌入苯基部分。两种芳炔阻转异构体都允许基于标准芳炔化学的完全对映特异性和可能的整体对映选择性合成原始阻转异构体。例如,与蒽和苝的反应分别以高对映体过量提供三蝶烯和纳米石墨烯阻转异构体。描述了由 BINOL 的任一对映异构体制备的双(芳烃)阻转异构体合成等效物,用于具有单手性的 3D 双向反应。ghi ]perylene) (98% ee) 纳米石墨烯阻转异构体。这允许发现双(twistacene)阻转异构体作为一类具有多重立体异构性的新型多环芳烃(PAH)。与双(芳烃)阻转异构体合成等价物和两种不同的亲吻化合物的交叉反应被证明是可行的,提供了一种具有苯并三蝶烯单元和蒽[1,2,3,4- ghi ]苝单元的纳米石墨烯阻转异构体,该单元围绕立体异构轴组装,作为独特的手性 PAH (99% ee)。总的来说,由于概念简单且易于实施,芳炔阻转异构体是广义上阻转异构体合成的一种有吸引力的方法。应用于合成具有单手性的大型 PAH 阻转异构体特别有前途。































 京公网安备 11010802027423号
京公网安备 11010802027423号