Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carrier-Free Immunotherapeutic Nano-Booster with Dual Synergistic Effects Based on Glutaminase Inhibition Combined with Photodynamic Therapy
ACS Nano ( IF 15.8 ) Pub Date : 2023-01-03 , DOI: 10.1021/acsnano.2c11037 Ziyi Mai 1, 2 , Jing Zhong 3 , Jiasi Zhang 2, 4 , Guimei Chen 1, 2 , Yan Tang 1, 2 , Wen Ma 1, 2 , Guang Li 1 , Zhenzhen Feng 1, 2 , Fangzhou Li 5 , Xing-Jie Liang 5, 6 , Yuanyuan Yang 1, 2 , Zhiqiang Yu 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2023-01-03 , DOI: 10.1021/acsnano.2c11037 Ziyi Mai 1, 2 , Jing Zhong 3 , Jiasi Zhang 2, 4 , Guimei Chen 1, 2 , Yan Tang 1, 2 , Wen Ma 1, 2 , Guang Li 1 , Zhenzhen Feng 1, 2 , Fangzhou Li 5 , Xing-Jie Liang 5, 6 , Yuanyuan Yang 1, 2 , Zhiqiang Yu 1, 2
Affiliation
|
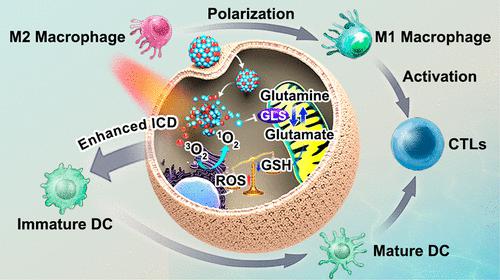
The immunotherapeutic effect elicited by photodynamic therapy (PDT) is attenuated by tumor defense mechanisms associated with glutamine metabolism, including the metabolic regulation of redox homeostasis and the limitation of the immunosuppressive tumor microenvironment (ITM). Herein, a carrier-free immunotherapeutic nanobooster C9SN with dual synergistic effects was constructed by the self-assembly of glutaminase (GLS) inhibitor compound 968 (C968) and photosensitizer Chlorin e6. C968-mediated GSH deprivation through inhibiting glutamine metabolism prevented PDT-generated reactive oxygen species from being annihilated by GSH, amplifying intracellular oxidative stress, which caused severe cell death and also enhanced the immunogenic cell death (ICD) effect. In addition, genome-wide analysis was carried out using RNA-sequencing to evaluate the changes in cell transcriptome induced by amplifying oxidative stress. Thereafter, neoantigens generated by the enhanced ICD effect promoted the maturation of dendritic cells, thereby recruiting and activating cytotoxic T lymphocytes (CTLs). Meanwhile, C9SN remodeled the ITM by blocking glutamine metabolism to polarize M2-type tumor-associated macrophages (TAMs) into M1-type TAMs, which further recruited and activated the CTLs. Ultimately, this immunotherapeutic nanobooster suppressed primary and distant tumors. This “kill two birds with one stone” strategy would shed light on enhancing tumor immunogenicity and alleviating tumor immunosuppression to improve the immunotherapeutic effect of PDT.
中文翻译:

基于谷氨酰胺酶抑制和光动力疗法双重协同作用的无载体免疫治疗纳米增强剂
光动力疗法(PDT)引起的免疫治疗效果因与谷氨酰胺代谢相关的肿瘤防御机制而减弱,包括氧化还原稳态的代谢调节和免疫抑制性肿瘤微环境(ITM)的限制。在此,通过谷氨酰胺酶(GLS)抑制剂化合物968(C968)和光敏剂Chlorin e6的自组装,构建了具有双重协同作用的无载体免疫治疗纳米增强剂C9SN。 C968通过抑制谷氨酰胺代谢介导的GSH剥夺,阻止PDT产生的活性氧被GSH消灭,放大细胞内氧化应激,导致严重的细胞死亡,并增强免疫原性细胞死亡(ICD)效应。此外,利用RNA测序进行全基因组分析,以评估氧化应激放大引起的细胞转录组变化。此后,增强的ICD效应产生的新抗原促进树突状细胞的成熟,从而募集并激活细胞毒性T淋巴细胞(CTL)。同时,C9SN通过阻断谷氨酰胺代谢来重塑ITM,将M2型肿瘤相关巨噬细胞(TAM)极化为M1型TAM,从而进一步招募并激活CTL。最终,这种免疫治疗纳米增强剂抑制了原发肿瘤和远处肿瘤。这种“一石二鸟”的策略将有助于增强肿瘤免疫原性,减轻肿瘤免疫抑制,从而提高PDT的免疫治疗效果。
更新日期:2023-01-03
中文翻译:

基于谷氨酰胺酶抑制和光动力疗法双重协同作用的无载体免疫治疗纳米增强剂
光动力疗法(PDT)引起的免疫治疗效果因与谷氨酰胺代谢相关的肿瘤防御机制而减弱,包括氧化还原稳态的代谢调节和免疫抑制性肿瘤微环境(ITM)的限制。在此,通过谷氨酰胺酶(GLS)抑制剂化合物968(C968)和光敏剂Chlorin e6的自组装,构建了具有双重协同作用的无载体免疫治疗纳米增强剂C9SN。 C968通过抑制谷氨酰胺代谢介导的GSH剥夺,阻止PDT产生的活性氧被GSH消灭,放大细胞内氧化应激,导致严重的细胞死亡,并增强免疫原性细胞死亡(ICD)效应。此外,利用RNA测序进行全基因组分析,以评估氧化应激放大引起的细胞转录组变化。此后,增强的ICD效应产生的新抗原促进树突状细胞的成熟,从而募集并激活细胞毒性T淋巴细胞(CTL)。同时,C9SN通过阻断谷氨酰胺代谢来重塑ITM,将M2型肿瘤相关巨噬细胞(TAM)极化为M1型TAM,从而进一步招募并激活CTL。最终,这种免疫治疗纳米增强剂抑制了原发肿瘤和远处肿瘤。这种“一石二鸟”的策略将有助于增强肿瘤免疫原性,减轻肿瘤免疫抑制,从而提高PDT的免疫治疗效果。































 京公网安备 11010802027423号
京公网安备 11010802027423号