当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Photocatalytic Synthesis of Acetic Acid from Methane and CO at Ambient Temperature Using Water as Oxidant
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-02 , DOI: 10.1021/jacs.2c10840 Chunyang Dong 1 , Maya Marinova 2 , Karima Ben Tayeb 3 , Olga V Safonova 4 , Yong Zhou 1 , Di Hu 1 , Sergei Chernyak 1 , Massimo Corda 1 , Jérémie Zaffran 1 , Andrei Y Khodakov 1 , Vitaly V Ordomsky 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-01-02 , DOI: 10.1021/jacs.2c10840 Chunyang Dong 1 , Maya Marinova 2 , Karima Ben Tayeb 3 , Olga V Safonova 4 , Yong Zhou 1 , Di Hu 1 , Sergei Chernyak 1 , Massimo Corda 1 , Jérémie Zaffran 1 , Andrei Y Khodakov 1 , Vitaly V Ordomsky 1
Affiliation
|
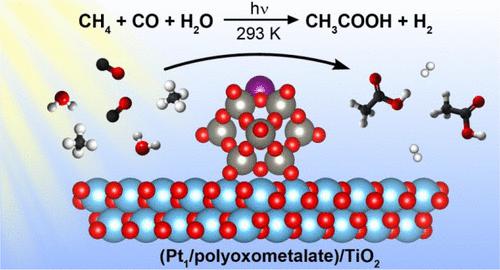
Direct functionalization of methane selectively to value-added chemicals is still one of the main challenges in modern science. Acetic acid is an important industrial chemical produced nowadays by expensive and environmentally unfriendly carbonylation of methanol using homogeneous catalysts. Here, we report a new photocatalytic reaction route to synthesize acetic acid from CH4 and CO at room temperature using water as the sole external oxygen source. The optimized photocatalyst consists of a TiO2 support and ammonium phosphotungstic polyoxometalate (NPW) clusters anchored with isolated Pt single atoms (Pt1). It enables a stable synthesis of 5.7 mmol·L–1 acetic acid solution in 60 h with the selectivity over 90% and 66% to acetic acid on liquid-phase and carbon basis, respectively, with the production of 99 mol of acetic acid per mol of Pt. Combined isotopic and in situ spectroscopy investigation suggests that synthesis of acetic acid proceeds via a photocatalytic oxidative carbonylation of methane over the Pt1 sites, with the methane activation facilitated by water-derived hydroxyl radicals.
中文翻译:

以水为氧化剂常温下甲烷和CO直接光催化合成乙酸
将甲烷选择性地直接官能化为增值化学品仍然是现代科学的主要挑战之一。乙酸是当今重要的工业化学品,通过使用均相催化剂对甲醇进行昂贵且对环境不友好的羰基化生产。在这里,我们报告了一种新的光催化反应路线,使用水作为唯一的外部氧源,在室温下从 CH 4和 CO合成乙酸。优化后的光催化剂由 TiO 2载体和固定有孤立 Pt 单原子 (Pt 1 ) 的磷钨多金属氧酸盐 (NPW) 簇组成。可稳定合成5.7 mmol·L –1乙酸溶液在 60 小时内在液相和碳基础上对乙酸的选择性分别超过 90% 和 66%,每 mol Pt 产生 99 mol 乙酸。结合同位素和原位光谱研究表明,乙酸的合成是通过甲烷在 Pt 1位点上的光催化氧化羰基化进行的,甲烷活化由水衍生的羟基自由基促进。
更新日期:2023-01-02
中文翻译:

以水为氧化剂常温下甲烷和CO直接光催化合成乙酸
将甲烷选择性地直接官能化为增值化学品仍然是现代科学的主要挑战之一。乙酸是当今重要的工业化学品,通过使用均相催化剂对甲醇进行昂贵且对环境不友好的羰基化生产。在这里,我们报告了一种新的光催化反应路线,使用水作为唯一的外部氧源,在室温下从 CH 4和 CO合成乙酸。优化后的光催化剂由 TiO 2载体和固定有孤立 Pt 单原子 (Pt 1 ) 的磷钨多金属氧酸盐 (NPW) 簇组成。可稳定合成5.7 mmol·L –1乙酸溶液在 60 小时内在液相和碳基础上对乙酸的选择性分别超过 90% 和 66%,每 mol Pt 产生 99 mol 乙酸。结合同位素和原位光谱研究表明,乙酸的合成是通过甲烷在 Pt 1位点上的光催化氧化羰基化进行的,甲烷活化由水衍生的羟基自由基促进。































 京公网安备 11010802027423号
京公网安备 11010802027423号