European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-12-30 , DOI: 10.1016/j.ejmech.2022.115072
Chao Wang 1 , Cangxin Zheng 1 , Han Wang 1 , Sufang Shui 2 , Hongwei Jin 3 , Guoquan Liu 2 , Fengrong Xu 1 , Zhenming Liu 4 , Liangren Zhang 4 , Dan Sun 5 , Ping Xu 1
|
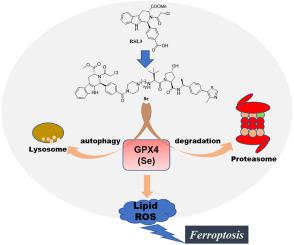
Targeting Glutathione peroxidase 4 (GPX4) has become a promising strategy for drug-resistant cancer therapy via ferroptosis induction. It was found that the GPX4 inhibitors such as RSL3 have GPX4 degradation ability via not only autophagy-lysosome pathway but also ubiquitin-proteasome system (UPS). Proteolysis targeting chimeras (PROTACs) using small molecule with both inhibition and degradation ability as the ligand of protein of interest (POI) have not been reported. To obtain better compounds with effective disturbance of GPX4 activity, and compare the difference between GPX4 inhibitors with degradation ability and their related PROTACs, we designed and synthesized a series of GPX4 degraders using PROTAC technology in terms of its excellent characteristics such as high efficiency and selectivity and the capacity of overcoming resistance. Hence, 8e was discovered as a potent and highly efficacious GPX4 degrader based upon the inhibitor RSL3. It was 2–3 times more potent than RSL3 in all the in vitro anti-tumor assays, indicating the importance of the PROTAC ternary complex of GPX4, 8e and E3 ligase ligand. 8e revealed better potency in resistant tumor cells than in wide type cells. Furthermore, we discovered for the first time that degrader 8e exhibit GPX4 degradation activity via ubiquitin-proteasome system (UPS) and autophagy-lysosome pathway with UPS plays the major role in the process. Our data also suggested that 8e and RSL3 could potently induce ferroptosis of HT1080 cells via GPX4 inhibition and degradation. In summary, our data revealed that the GPX4 degrader 8e achieves better degradation and anti-tumor effects compared to its related GPX4 inhibitor RSL3. Thus, an efficient strategy to induce GPX4 degradation and subsequent ferroptosis was established in this study for malignant cancer treatment in the future.
中文翻译:

GPX4降解剂双重降解机制诱导铁死亡发挥抗肿瘤作用
靶向谷胱甘肽过氧化物酶 4 (GPX4) 已成为通过铁死亡诱导进行耐药性癌症治疗的一种有前途的策略。研究发现,RSL3 等 GPX4 抑制剂不仅可以通过自噬-溶酶体途径,还可以通过泛素-蛋白酶体系统 (UPS) 降解 GPX4。使用具有抑制和降解能力的小分子作为感兴趣的蛋白质 (POI) 的配体的蛋白水解靶向嵌合体 (PROTAC) 尚未见报道。为了获得更好的有效干扰GPX4活性的化合物,并比较具有降解能力的GPX4抑制剂与其相关PROTAC的差异,我们利用PROTAC技术设计合成了一系列具有高效和选择性等优良特性的GPX4降解剂以及克服阻力的能力。因此,8e被发现是一种基于抑制剂 RSL3 的有效且高效的 GPX4 降解剂。在所有体外抗肿瘤试验中,它的效力是 RSL3 的 2-3 倍,表明GPX4、8e和 E3 连接酶配体的 PROTAC 三元复合物的重要性。8e显示在抗性肿瘤细胞中比在宽型细胞中具有更好的效力。此外,我们首次发现降解剂8e通过泛素-蛋白酶体系统 (UPS) 表现出 GPX4 降解活性,而 UPS 的自噬-溶酶体途径在该过程中起主要作用。我们的数据还表明8eRSL3 可通过 GPX4 抑制和降解有效诱导 HT1080 细胞铁死亡。总之,我们的数据显示,与其相关的 GPX4 抑制剂 RSL3 相比,GPX4 降解剂8e具有更好的降解和抗肿瘤效果。因此,本研究建立了一种诱导 GPX4 降解和随后的铁死亡的有效策略,用于未来的恶性肿瘤治疗。

































 京公网安备 11010802027423号
京公网安备 11010802027423号