当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Contrasting Mechanisms of Aromatic and Aryl-Methyl Substituent Hydroxylation by the Rieske Monooxygenase Salicylate 5-Hydroxylase
Biochemistry ( IF 2.9 ) Pub Date : 2022-12-30 , DOI: 10.1021/acs.biochem.2c00610 Melanie S Rogers 1 , Adrian M Gordon 2 , Todd M Rappe 3 , Jason D Goodpaster 2 , John D Lipscomb 1
Biochemistry ( IF 2.9 ) Pub Date : 2022-12-30 , DOI: 10.1021/acs.biochem.2c00610 Melanie S Rogers 1 , Adrian M Gordon 2 , Todd M Rappe 3 , Jason D Goodpaster 2 , John D Lipscomb 1
Affiliation
|
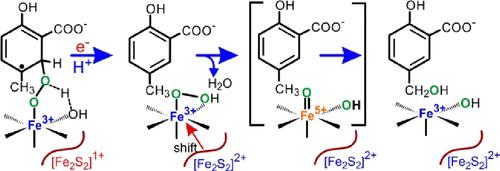
The hydroxylase component (S5HH) of salicylate-5-hydroxylase catalyzes C5 ring hydroxylation of salicylate but switches to methyl hydroxylation when a C5 methyl substituent is present. The use of 18O2 reveals that both aromatic and aryl-methyl hydroxylations result from monooxygenase chemistry. The functional unit of S5HH comprises a nonheme Fe(II) site located 12 Å across a subunit boundary from a one-electron reduced Rieske-type iron–sulfur cluster. Past studies determined that substrates bind near the Fe(II), followed by O2 binding to the iron to initiate catalysis. Stopped-flow-single-turnover reactions (STOs) demonstrated that the Rieske cluster transfers an electron to the iron site during catalysis. It is shown here that fluorine ring substituents decrease the rate constant for Rieske electron transfer, implying a prior reaction of an Fe(III)-superoxo intermediate with a substrate. We propose that the iron becomes fully oxidized in the resulting Fe(III)-peroxo-substrate-radical intermediate, allowing Rieske electron transfer to occur. STO using 5-CD3-salicylate-d8 occurs with an inverse kinetic isotope effect (KIE). In contrast, STO of a 1:1 mixture of unlabeled and 5-CD3-salicylate-d8 yields a normal product isotope effect. It is proposed that aromatic and aryl-methyl hydroxylation reactions both begin with the Fe(III)-superoxo reaction with a ring carbon, yielding the inverse KIE due to sp2 → sp3 carbon hybridization. After Rieske electron transfer, the resulting Fe(III)-peroxo-salicylate intermediate can continue to aromatic hydroxylation, whereas the equivalent aryl-methyl intermediate formation must be reversible to allow the substrate exchange necessary to yield a normal product isotope effect. The resulting Fe(III)-(hydro)peroxo intermediate may be reactive or evolve through a high-valent iron intermediate to complete the aryl-methyl hydroxylation.
中文翻译:

Rieske 单加氧酶水杨酸 5-羟化酶对芳香族和芳基甲基取代基羟基化的对比机制
水杨酸-5-羟化酶的羟化酶成分 (S5HH) 催化水杨酸的 C5 环羟基化,但当存在 C5 甲基取代基时转为甲基羟基化。 18 O 2的使用表明芳香族羟基化和芳基甲基羟基化都是由单加氧酶化学作用产生的。 S5HH 的功能单元包含一个非血红素 Fe(II) 位点,位于距单电子还原 Rieske 型铁硫簇的亚基边界 12 Å 处。过去的研究确定底物在 Fe(II) 附近结合,然后 O 2与铁结合以启动催化。停流单周转反应 (STO) 证明 Rieske 簇在催化过程中将电子转移到铁位点。这里表明,氟环取代基降低了 Rieske 电子转移的速率常数,这意味着 Fe(III)-超氧中间体与底物的预先反应。我们提出,铁在所得的 Fe(III)-过氧-底物-自由基中间体中被完全氧化,从而允许发生 Rieske 电子转移。使用 5-CD 3 -水杨酸盐-d 8的 STO 发生逆动力同位素效应 (KIE)。相反,未标记和 5-CD 3 -水杨酸-d 8的 1:1 混合物的 STO 产生正常的产物同位素效应。据推测,芳香族和芳基甲基羟基化反应均始于与环碳的 Fe(III)-超氧反应,由于 sp 2 → sp 3碳杂化而产生逆 KIE。 Rieske电子转移后,生成的Fe(III)-过氧水杨酸酯中间体可以继续芳香族羟基化,而等效的芳基-甲基中间体的形成必须是可逆的,以允许产生正常产物同位素效应所需的底物交换。所得的 Fe(III)-(氢)过氧中间体可以是反应性的或通过高价铁中间体放出以完成芳基-甲基羟基化。
更新日期:2022-12-30
中文翻译:

Rieske 单加氧酶水杨酸 5-羟化酶对芳香族和芳基甲基取代基羟基化的对比机制
水杨酸-5-羟化酶的羟化酶成分 (S5HH) 催化水杨酸的 C5 环羟基化,但当存在 C5 甲基取代基时转为甲基羟基化。 18 O 2的使用表明芳香族羟基化和芳基甲基羟基化都是由单加氧酶化学作用产生的。 S5HH 的功能单元包含一个非血红素 Fe(II) 位点,位于距单电子还原 Rieske 型铁硫簇的亚基边界 12 Å 处。过去的研究确定底物在 Fe(II) 附近结合,然后 O 2与铁结合以启动催化。停流单周转反应 (STO) 证明 Rieske 簇在催化过程中将电子转移到铁位点。这里表明,氟环取代基降低了 Rieske 电子转移的速率常数,这意味着 Fe(III)-超氧中间体与底物的预先反应。我们提出,铁在所得的 Fe(III)-过氧-底物-自由基中间体中被完全氧化,从而允许发生 Rieske 电子转移。使用 5-CD 3 -水杨酸盐-d 8的 STO 发生逆动力同位素效应 (KIE)。相反,未标记和 5-CD 3 -水杨酸-d 8的 1:1 混合物的 STO 产生正常的产物同位素效应。据推测,芳香族和芳基甲基羟基化反应均始于与环碳的 Fe(III)-超氧反应,由于 sp 2 → sp 3碳杂化而产生逆 KIE。 Rieske电子转移后,生成的Fe(III)-过氧水杨酸酯中间体可以继续芳香族羟基化,而等效的芳基-甲基中间体的形成必须是可逆的,以允许产生正常产物同位素效应所需的底物交换。所得的 Fe(III)-(氢)过氧中间体可以是反应性的或通过高价铁中间体放出以完成芳基-甲基羟基化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号