Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous FeIII/FeII redox cycling in single-atom catalysts: Conjugation effect and electron delocalization
iScience ( IF 4.6 ) Pub Date : 2022-12-28 , DOI: 10.1016/j.isci.2022.105902
Zheng Qian 1 , Lingzhen Wang 1 , Mawuli Dzakpasu 1 , Yujia Tian 1 , Dahu Ding 2 , Rongzhi Chen 3 , Gen Wang 1 , Shengjiong Yang 1
iScience ( IF 4.6 ) Pub Date : 2022-12-28 , DOI: 10.1016/j.isci.2022.105902
Zheng Qian 1 , Lingzhen Wang 1 , Mawuli Dzakpasu 1 , Yujia Tian 1 , Dahu Ding 2 , Rongzhi Chen 3 , Gen Wang 1 , Shengjiong Yang 1
Affiliation
|
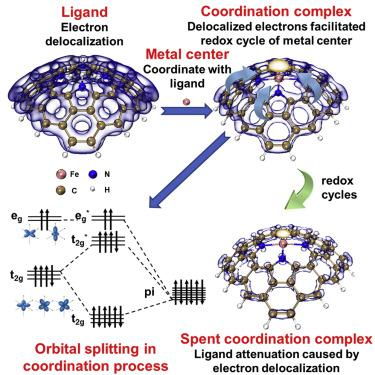
The mechanism of spontaneous Fe/Fe redox cycling in iron-centered single-atom catalysts (I-SACs) is often overlooked. Consequently, pathways for continuous SO/HO⋅ generation during peroxymonosulfate (PMS) activation by I-SACs remain unclear. Herein, the evolution of the iron center and ligand in I-SACs was comprehensively investigated. I-SACs could be considered as a coordination complex created by iron and a heteroatom N-doped carbonaceous ligand. The ligand-field theory could well explain the electronic behavior of the complex, whereby electrons delocalized by the conjugation effect of the ligand were confirmed to be responsible for the Fe/Fe redox cycle. The possible pyridinic ligand in I-SACs was demonstrably weaker than the pyrrolic ligand in Fe reduction due to its shielding effect on delocalized π orbitals by local lone-pair electrons. The results of this study significantly advance our understanding of the mechanism of spontaneous Fe/Fe redox cycling and radical generation pathways in the I-SACs/PMS process.
中文翻译:

单原子催化剂中的自发 FeIII/FeII 氧化还原循环:共轭效应和电子离域
以铁为中心的单原子催化剂(I-SAC)中自发的 Fe/Fe 氧化还原循环机制经常被忽视。因此,I-SAC 激活过一硫酸盐 (PMS) 期间连续生成 SO/H2O· 的途径仍不清楚。在此,全面研究了 I-SAC 中铁中心和配体的演化。 I-SACs 可以被认为是由铁和杂原子氮掺杂碳质配体产生的配位络合物。配体场理论可以很好地解释配合物的电子行为,其中通过配体的共轭效应而离域的电子被证实负责Fe/Fe氧化还原循环。 I-SAC 中可能的吡啶配体在 Fe 还原中明显弱于吡咯配体,因为其对局域孤对电子对离域 π 轨道的屏蔽作用。这项研究的结果显着增进了我们对 I-SACs/PMS 过程中自发 Fe/Fe 氧化还原循环机制和自由基生成途径的理解。
更新日期:2022-12-28
中文翻译:

单原子催化剂中的自发 FeIII/FeII 氧化还原循环:共轭效应和电子离域
以铁为中心的单原子催化剂(I-SAC)中自发的 Fe/Fe 氧化还原循环机制经常被忽视。因此,I-SAC 激活过一硫酸盐 (PMS) 期间连续生成 SO/H2O· 的途径仍不清楚。在此,全面研究了 I-SAC 中铁中心和配体的演化。 I-SACs 可以被认为是由铁和杂原子氮掺杂碳质配体产生的配位络合物。配体场理论可以很好地解释配合物的电子行为,其中通过配体的共轭效应而离域的电子被证实负责Fe/Fe氧化还原循环。 I-SAC 中可能的吡啶配体在 Fe 还原中明显弱于吡咯配体,因为其对局域孤对电子对离域 π 轨道的屏蔽作用。这项研究的结果显着增进了我们对 I-SACs/PMS 过程中自发 Fe/Fe 氧化还原循环机制和自由基生成途径的理解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号