当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acyl azide modification of the ubiquitin C-terminus enables DUB capture
Chemical Communications ( IF 4.3 ) Pub Date : 2022-12-29 , DOI: 10.1039/d2cc06496k Xiao Hua 1 , Yanyan Guo 1 , Yu Wang 1 , Guo-Chao Chu 2 , Pincheng Li 3 , Jing Shi 1
Chemical Communications ( IF 4.3 ) Pub Date : 2022-12-29 , DOI: 10.1039/d2cc06496k Xiao Hua 1 , Yanyan Guo 1 , Yu Wang 1 , Guo-Chao Chu 2 , Pincheng Li 3 , Jing Shi 1
Affiliation
|
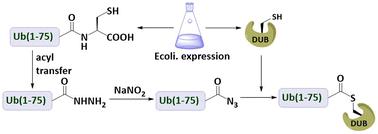
Deubiquitinating enzyme (DUB) abnormalities are associated with many diseases. Previous attempts have been made to introduce various chemical groups such as alkynes, unsaturated olefins and alkyl halides to the C-terminus of ubiquitin (Ub) to capture the active-site cysteine residue in DUBs for structural and biochemical studies. Here, we find that a Ub C-terminal acyl azide can capture DUBs, thereby forming thioester bonds in buffers and cell lysates. This finding not only makes ubiquitin acyl azide a chemical probe for capturing DUBs, but also extends the utility of azide groups in biological applications.
中文翻译:

泛素 C 端的酰基叠氮化物修饰使 DUB 捕获成为可能
去泛素化酶 (DUB) 异常与许多疾病有关。先前已尝试将各种化学基团(如炔烃、不饱和烯烃和卤代烷)引入泛素 (Ub) 的 C 末端,以捕获 DUB 中的活性位点半胱氨酸残基,用于结构和生化研究。在这里,我们发现 Ub C 端酰基叠氮化物可以捕获 DUB,从而在缓冲液和细胞裂解物中形成硫酯键。这一发现不仅使泛素酰基叠氮化物成为捕获 DUB 的化学探针,而且扩展了叠氮基团在生物学应用中的效用。
更新日期:2022-12-29
中文翻译:

泛素 C 端的酰基叠氮化物修饰使 DUB 捕获成为可能
去泛素化酶 (DUB) 异常与许多疾病有关。先前已尝试将各种化学基团(如炔烃、不饱和烯烃和卤代烷)引入泛素 (Ub) 的 C 末端,以捕获 DUB 中的活性位点半胱氨酸残基,用于结构和生化研究。在这里,我们发现 Ub C 端酰基叠氮化物可以捕获 DUB,从而在缓冲液和细胞裂解物中形成硫酯键。这一发现不仅使泛素酰基叠氮化物成为捕获 DUB 的化学探针,而且扩展了叠氮基团在生物学应用中的效用。































 京公网安备 11010802027423号
京公网安备 11010802027423号