当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trojan Horse Nanocapsule Enabled In Situ Modulation of the Phenotypic Conversion of Th17 Cells to Treg Cells for the Treatment of Multiple Sclerosis in Mice
Advanced Materials ( IF 27.4 ) Pub Date : 2022-12-27 , DOI: 10.1002/adma.202210262 Chongdeng Shi 1 , Jing Zhang 1 , Huijun Wang 2 , Chen Chen 1 , Maosen Han 1 , Lin Gao 1 , Chunwei Tang 1 , Peng Sun 3 , Xiaotian Zhao 1 , Feiyue Guo 1 , Zhaozhong Wang 1 , Mohnad Abdalla 1 , Zhenmei Yang 1 , Ying Liu 1 , Anning Li 2 , Cai Zhang 1 , Xinyi Jiang 1
Advanced Materials ( IF 27.4 ) Pub Date : 2022-12-27 , DOI: 10.1002/adma.202210262 Chongdeng Shi 1 , Jing Zhang 1 , Huijun Wang 2 , Chen Chen 1 , Maosen Han 1 , Lin Gao 1 , Chunwei Tang 1 , Peng Sun 3 , Xiaotian Zhao 1 , Feiyue Guo 1 , Zhaozhong Wang 1 , Mohnad Abdalla 1 , Zhenmei Yang 1 , Ying Liu 1 , Anning Li 2 , Cai Zhang 1 , Xinyi Jiang 1
Affiliation
|
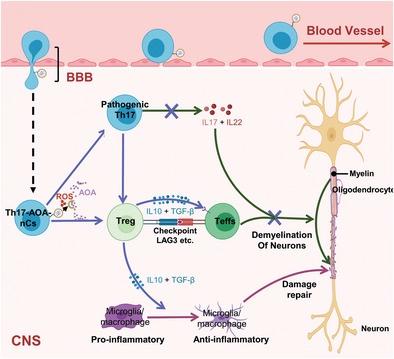
Th17/Treg imbalance is closely related to the occurrence and development of multiple sclerosis (MS), and the transdifferentiation of Th17 cells into Treg cells may contribute to the resolution of inflammation, presenting a therapeutic strategy for MS. To modulate this phenotypic shift in situ, a “Trojan horse”-like hybrid system, nanocapsule-coupled Th17 cells, is reported for MS treatment. Following intravenous injection into MS mice, the hybrid system efficiently transmigrates across the blood–brain barrier and homes to the inflamed MS niche. (Aminooxy)-acetic acid, a transdifferentiation inducer, is locally released upon the production of ROS and in turn taken up by Th17 cells. It is demonstrated that the Trojan horse hybrid system enables in situ phenotypic transdifferentiation of Th17 cells into anti-inflammatory Treg cells. This phenotypic conversion leads to a domino-like immune response that is conducive to MS therapy. Overall, this work highlights a new pathway for accurate modulation of the phenotypes of adoptively transferred cells in situ, from proinflammatory to anti-inflammatory for MS therapy, and may be broadly applicable for patients suffering from other autoimmune diseases.
中文翻译:

特洛伊木马纳米胶囊能够原位调节 Th17 细胞向 Treg 细胞的表型转化,用于治疗小鼠多发性硬化症
Th17/Treg失衡与多发性硬化症(MS)的发生发展密切相关,Th17细胞向Treg细胞的转分化可能有助于炎症的消退,为MS提供了治疗策略。为了在原位调节这种表型转变,据报道一种“特洛伊木马”样混合系统,即纳米胶囊偶联的 Th17 细胞用于 MS 治疗。在对 MS 小鼠进行静脉注射后,混合系统有效地穿过血脑屏障并回到发炎的 MS 生态位。(氨氧基)-乙酸是一种转分化诱导剂,在产生 ROS 时局部释放,然后被 Th17 细胞吸收。结果表明,特洛伊木马杂交系统能够使 Th17 细胞原位表型转分化为抗炎 Treg 细胞。这种表型转换导致多米诺骨牌样免疫反应,有利于 MS 治疗。总的来说,这项工作强调了一种新途径,用于准确调节原位过继转移细胞的表型,从促炎到抗炎治疗 MS,并可能广泛适用于患有其他自身免疫性疾病的患者。
更新日期:2022-12-27
中文翻译:

特洛伊木马纳米胶囊能够原位调节 Th17 细胞向 Treg 细胞的表型转化,用于治疗小鼠多发性硬化症
Th17/Treg失衡与多发性硬化症(MS)的发生发展密切相关,Th17细胞向Treg细胞的转分化可能有助于炎症的消退,为MS提供了治疗策略。为了在原位调节这种表型转变,据报道一种“特洛伊木马”样混合系统,即纳米胶囊偶联的 Th17 细胞用于 MS 治疗。在对 MS 小鼠进行静脉注射后,混合系统有效地穿过血脑屏障并回到发炎的 MS 生态位。(氨氧基)-乙酸是一种转分化诱导剂,在产生 ROS 时局部释放,然后被 Th17 细胞吸收。结果表明,特洛伊木马杂交系统能够使 Th17 细胞原位表型转分化为抗炎 Treg 细胞。这种表型转换导致多米诺骨牌样免疫反应,有利于 MS 治疗。总的来说,这项工作强调了一种新途径,用于准确调节原位过继转移细胞的表型,从促炎到抗炎治疗 MS,并可能广泛适用于患有其他自身免疫性疾病的患者。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号