Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic Acid Acetoxymethyl Ester Loaded Reactive Oxygen Species Responsive Hyaluronic Acid–Bilirubin Nanoparticles for Acute Kidney Injury Therapy via Alleviating Calcium Overload Mediated Endoplasmic Reticulum Stress
ACS Nano ( IF 15.8 ) Pub Date : 2022-12-27 , DOI: 10.1021/acsnano.2c08982 Yanan Wang 1, 2 , Minju Pu 1, 2 , Jiahui Yan 1, 2 , Jingwen Zhang 1, 2 , Huichao Wei 1, 2 , Liangmin Yu 1, 2 , Xuefeng Yan 1, 2 , Zhiyu He 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2022-12-27 , DOI: 10.1021/acsnano.2c08982 Yanan Wang 1, 2 , Minju Pu 1, 2 , Jiahui Yan 1, 2 , Jingwen Zhang 1, 2 , Huichao Wei 1, 2 , Liangmin Yu 1, 2 , Xuefeng Yan 1, 2 , Zhiyu He 1, 2
Affiliation
|
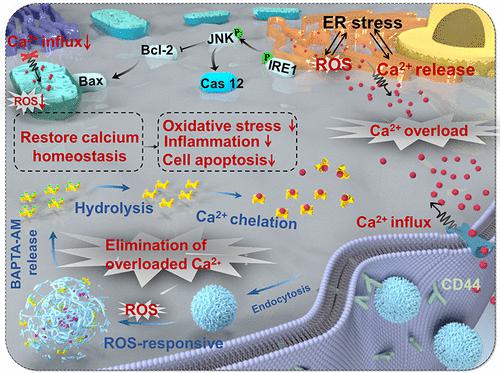
Calcium overload is one of the early determinants of the core cellular events that contribute to the pathogenesis of acute kidney injury (AKI), which include oxidative stress, ATP depletion, calcium overload, and inflammatory response with self-amplifying and interactive feedback loops that ultimately lead to cellular injury and renal failure. Excluding adjuvant therapy, there are currently no approved pharmacotherapies for the treatment of AKI. Using an adipic dihydride linker, we modified the hyaluronic acid polymer chain with a potent antioxidant, bilirubin, to produce an amphiphilic conjugate. Subsequently, we developed a kidney-targeted and reactive oxygen species (ROS)-responsive drug delivery system based on the flash nanocomplexation method to deliver a well-known intracellular calcium chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM, BA), with the goal of rescuing renal cell damage via rapidly scavenging of intracellularly overloaded Ca2+. In the ischemia–reperfusion (I/R) induced AKI rat model, a single dose of as-prepared formulation (BA 100 μg·kg–1) 6 h post-reperfusion significantly reduced renal function indicators by more than 60% within 12 h, significantly alleviated tissular pathological changes, ameliorated tissular oxidative damage, significantly inhibited apoptosis of renal tubular cells and the expression of renal tubular marker kidney injury molecule 1, etc., thus greatly reducing the risk of kidney failure. Mechanistically, the treatment with BA-loaded NPs significantly inhibited the activation of the ER stress cascade response (IRE1-TRAF2-JNK, ATF4-CHOP, and ATF6 axis) and regulated the downstream apoptosis-related pathway while also reducing the inflammatory response. The BA-loaded NPs hold great promise as a potential therapy for I/R injury-related diseases.
中文翻译:

1,2-双(2-氨基苯氧基)乙烷-N,N,N',N'-四乙酸乙酰氧基甲基酯负载活性氧物质反应性透明质酸-胆红素纳米颗粒通过减轻钙过载介导的内质网应激治疗急性肾损伤
钙过载是导致急性肾损伤 (AKI) 发病机制的核心细胞事件的早期决定因素之一,这些事件包括氧化应激、ATP 耗竭、钙过载和具有自我放大和交互反馈循环的炎症反应,最终导致细胞损伤和肾功能衰竭。除辅助治疗外,目前尚无批准用于治疗 AKI 的药物疗法。我们使用己二酸二氢化物接头,用强效抗氧化剂胆红素修饰透明质酸聚合物链,以产生两亲性结合物。随后,我们开发了一种基于快速纳米络合方法的肾脏靶向和活性氧 (ROS) 响应药物递送系统,以递送众所周知的细胞内钙螯合剂 1,2-双(2-氨基苯氧基)乙烷-N , N , N ′, N ′-四乙酸乙酰氧基甲酯 (BAPTA-AM, BA),目的是通过快速清除细胞内超载的 Ca 2+来挽救肾细胞损伤。在缺血再灌注 (I/R) 诱导的 AKI 大鼠模型中,单剂量的制剂(BA 100 μg·kg –1)再灌注6 h后12 h内肾功能指标显着降低60%以上,组织病理改变明显减轻,组织氧化损伤改善,显着抑制肾小管细胞凋亡和肾小管标志性肾损伤分子1的表达,等,从而大大降低肾衰竭的风险。从机制上讲,负载 BA 的 NPs 处理显着抑制了 ER 应激级联反应(IRE1-TRAF2-JNK、ATF4-CHOP 和 ATF6 轴)的激活,并调节了下游细胞凋亡相关通路,同时也减少了炎症反应。负载 BA 的 NPs 作为 I/R 损伤相关疾病的潜在疗法具有很大的前景。
更新日期:2022-12-27
中文翻译:

1,2-双(2-氨基苯氧基)乙烷-N,N,N',N'-四乙酸乙酰氧基甲基酯负载活性氧物质反应性透明质酸-胆红素纳米颗粒通过减轻钙过载介导的内质网应激治疗急性肾损伤
钙过载是导致急性肾损伤 (AKI) 发病机制的核心细胞事件的早期决定因素之一,这些事件包括氧化应激、ATP 耗竭、钙过载和具有自我放大和交互反馈循环的炎症反应,最终导致细胞损伤和肾功能衰竭。除辅助治疗外,目前尚无批准用于治疗 AKI 的药物疗法。我们使用己二酸二氢化物接头,用强效抗氧化剂胆红素修饰透明质酸聚合物链,以产生两亲性结合物。随后,我们开发了一种基于快速纳米络合方法的肾脏靶向和活性氧 (ROS) 响应药物递送系统,以递送众所周知的细胞内钙螯合剂 1,2-双(2-氨基苯氧基)乙烷-N , N , N ′, N ′-四乙酸乙酰氧基甲酯 (BAPTA-AM, BA),目的是通过快速清除细胞内超载的 Ca 2+来挽救肾细胞损伤。在缺血再灌注 (I/R) 诱导的 AKI 大鼠模型中,单剂量的制剂(BA 100 μg·kg –1)再灌注6 h后12 h内肾功能指标显着降低60%以上,组织病理改变明显减轻,组织氧化损伤改善,显着抑制肾小管细胞凋亡和肾小管标志性肾损伤分子1的表达,等,从而大大降低肾衰竭的风险。从机制上讲,负载 BA 的 NPs 处理显着抑制了 ER 应激级联反应(IRE1-TRAF2-JNK、ATF4-CHOP 和 ATF6 轴)的激活,并调节了下游细胞凋亡相关通路,同时也减少了炎症反应。负载 BA 的 NPs 作为 I/R 损伤相关疾病的潜在疗法具有很大的前景。






























 京公网安备 11010802027423号
京公网安备 11010802027423号