当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvation regulation to mitigate the decomposition of 2,6-dihydroxyanthraquinone in aqueous organic redox flow batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-12-26 , DOI: 10.1039/d2ee03617g Kang Peng 1 , Yuanyuan Li 1 , Gonggen Tang 1 , Yahua Liu 2 , Zhengjin Yang 1 , Tongwen Xu 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-12-26 , DOI: 10.1039/d2ee03617g Kang Peng 1 , Yuanyuan Li 1 , Gonggen Tang 1 , Yahua Liu 2 , Zhengjin Yang 1 , Tongwen Xu 1
Affiliation
|
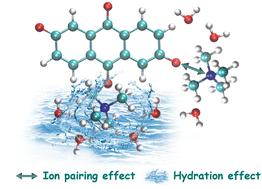
In the development of aqueous organic redox flow batteries (AORFBs), anthraquinone derivatives (AQs) attract a great deal of attention as the most promising negative electrolytes. The molecular structures of AQs have been engineered to maintain their long-term stability in aqueous media, which, however, requires tedious syntheses and multi-step purifications. Herein, we document the first case of a solvation regulation strategy to extend the lifetime of 2,6-dihydroxyanthraquinone (DHAQ) electrolytes, i.e., incorporating tetramethylammonium cations (TMA+) in the supporting electrolytes to interfere with the solvation structure of DHAQ2−/DHAHQ4− anions, thereby deactivating the chemical or electrochemical reduction of DHAHQ4− that initiates the subsequent side reactions. The ion pairing and hydration effect of TMA+ are elaborately demonstrated through experiments and simulations. The capacity fade rate of DHAQ/K4Fe(CN)6 cells caused by 0.1 M DHAQ electrolyte decomposition decreases by almost an order of magnitude, from 5.34% per day without TMA+ to 0.65% per day with 4.5 M TMA+. This strategy is effective when the DHAQ concentration is raised to 0.4 M. We anticipate that this mitigation strategy will readily extend to other organic redox-active molecules.
中文翻译:

溶剂化调节减轻 2,6-二羟基蒽醌在水性有机氧化还原液流电池中的分解
在水系有机氧化还原液流电池(AORFBs)的开发中,蒽醌衍生物(AQs)作为最有前途的负极电解质而备受关注。AQ 的分子结构经过精心设计,可在水性介质中保持长期稳定性,然而,这需要繁琐的合成和多步纯化。在此,我们记录了第一个延长 2,6-二羟基蒽醌 (DHAQ) 电解质寿命的溶剂化调节策略案例,即在支持电解质中加入四甲基铵阳离子 (TMA + ) 以干扰 DHAQ 2−的溶剂化结构/DHAHQ 4−阴离子,从而使 DHAHQ 的化学或电化学还原失活4−启动后续的副反应。TMA +的离子配对和水合作用通过实验和模拟得到了详尽的证明。由 0.1 M DHAQ 电解质分解引起的 DHAQ/K 4 Fe(CN) 6电池的容量衰减率几乎降低了一个数量级,从没有 TMA +的每天 5.34% 到有 4.5 M TMA +的每天 0.65%。当 DHAQ 浓度提高到 0.4 M 时,该策略是有效的。我们预计该缓解策略将很容易扩展到其他有机氧化还原活性分子。
更新日期:2022-12-26
中文翻译:

溶剂化调节减轻 2,6-二羟基蒽醌在水性有机氧化还原液流电池中的分解
在水系有机氧化还原液流电池(AORFBs)的开发中,蒽醌衍生物(AQs)作为最有前途的负极电解质而备受关注。AQ 的分子结构经过精心设计,可在水性介质中保持长期稳定性,然而,这需要繁琐的合成和多步纯化。在此,我们记录了第一个延长 2,6-二羟基蒽醌 (DHAQ) 电解质寿命的溶剂化调节策略案例,即在支持电解质中加入四甲基铵阳离子 (TMA + ) 以干扰 DHAQ 2−的溶剂化结构/DHAHQ 4−阴离子,从而使 DHAHQ 的化学或电化学还原失活4−启动后续的副反应。TMA +的离子配对和水合作用通过实验和模拟得到了详尽的证明。由 0.1 M DHAQ 电解质分解引起的 DHAQ/K 4 Fe(CN) 6电池的容量衰减率几乎降低了一个数量级,从没有 TMA +的每天 5.34% 到有 4.5 M TMA +的每天 0.65%。当 DHAQ 浓度提高到 0.4 M 时,该策略是有效的。我们预计该缓解策略将很容易扩展到其他有机氧化还原活性分子。































 京公网安备 11010802027423号
京公网安备 11010802027423号