当前位置:
X-MOL 学术
›
Chin. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel route for the synthesis of androgen receptor antagonist enzalutamide
Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2022-12-24 , DOI: 10.1016/j.cclet.2022.108096 Xiangguo Meng , Siju Bi , Shixin Jin , Kai Wu , Shanchao Wu , Lei Shao , Pierre-Antoine Bonnet , Chunquan Sheng
中文翻译:

雄激素受体拮抗剂恩杂鲁胺的合成新路线
更新日期:2022-12-24
Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2022-12-24 , DOI: 10.1016/j.cclet.2022.108096 Xiangguo Meng , Siju Bi , Shixin Jin , Kai Wu , Shanchao Wu , Lei Shao , Pierre-Antoine Bonnet , Chunquan Sheng
|
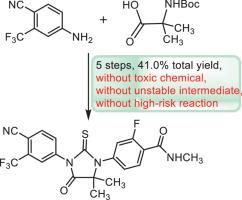
A novel route of enzalutamide was developed in five steps. Starting from 4-amino-2-(trifluoromethyl)benzonitrile (7) and Boc-2-aminoisobutyric acid (16), condensation, deprotection, Ullmann coupling, cyclization and amination provided enzalutamide in 41.0% total yield. This route avoids the using of toxic chemical, unstable intermediate and high-risk reaction. It is a potential efficient and economical procedure for industrialization.
中文翻译:

雄激素受体拮抗剂恩杂鲁胺的合成新路线
分五步开发了恩杂鲁胺的新路线。从 4-氨基-2-(三氟甲基) 苄腈 ( 7 ) 和 Boc-2-氨基异丁酸 ( 16 ) 开始,缩合、脱保护、Ullmann 偶联、环化和胺化以 41.0% 的总收率提供恩杂鲁胺。该路线避免了使用有毒化学品、不稳定的中间体和高危反应。这是一种潜在的高效和经济的工业化程序。































 京公网安备 11010802027423号
京公网安备 11010802027423号