Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2022-12-17 , DOI: 10.1016/j.colsurfa.2022.130798 Yun Sheng , Xueqian Zhang , Bo Lan , Chuncheng Wei , Yishan Wang , Guangwu Wen
|
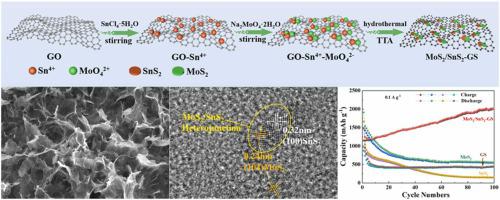
The specific capacity of MoS2 is much larger than that of commercial graphite, but it cannot be practically applied due to its poor electrical conductivity and low cycle stability. Although the conductivity of the material can be greatly increased after compounding with graphene, MoS2 will be unevenly distributed on graphene and form agglomeration because of the charge incompatibility caused by negatively charged molybdenum sources of GO and MoS2. Therefore, the capacity of the as-prepared MoS2/graphene material is extremely unstable and decays to a great extent during cycling. In this paper, a three-dimensional layered structure of MoS2/SnS2 and graphene (MoS2/SnS2-GS) as anode material for sulfur-doped lithium-ion batteries were prepared by a one-pot hydrothermal method. Sn4+ is a bridge connecting two anionic materials, neutralizes negatively charged GO through electrostatic interaction, and adsorbs MoO42-. Furthermore, the heterostructure of MoS2/SnS2 provides a faster and more efficient channel for Li+. Moreover, graphene with high surface area and excellent electrical conductivity allow the electrolyte to provide much fuller access to enter materials, which promotes the transport of Li+. Sulfur doping has a strong effect on surface activation, resulting in a significant increase in surface adsorption and chemical kinetics. Consequently, MoS2/SnS2-GS exhibits excellent capacity and cycling performance as anode material for Lithium-ion batteries (LIBs), maintaining a specific capacity of 2007.4 mAh g−1 at 0.1 A g−1 (100 cycles), and 3224.3 mAh g−1 at 0.5 A g−1 (600 cycles). The preparation method of MoS2/SnS2-GS material provides a great reference for the development of anode materials for disulfide LIBs.
中文翻译:

MoS2/SnS2协同石墨烯构建优质储锂负极材料
MoS 2的比容量远大于商业石墨,但由于其导电性差和循环稳定性低而无法实际应用。虽然与石墨烯复合后材料的导电性可以大大提高,但由于GO和MoS 2带负电荷的钼源造成的电荷不相容,MoS 2会在石墨烯上分布不均并形成团聚。因此,所制备的MoS 2 /石墨烯材料的容量极不稳定,在循环过程中衰减很大。在本文中,一种三维层状结构的MoS 2 /SnS 2和石墨烯(MoS 2/SnS 2 -GS)采用一锅法水热法制备了硫掺杂锂离子电池负极材料。Sn 4+是连接两种阴离子材料的桥梁,通过静电作用中和带负电荷的GO,吸附MoO 4 2-。此外,MoS 2 /SnS 2的异质结构为Li +提供了更快、更有效的通道。此外,具有高表面积和优异导电性的石墨烯使电解质能够更充分地进入材料,从而促进 Li +的传输. 硫掺杂对表面活化有很强的影响,导致表面吸附和化学动力学显着增加。因此,MoS 2 /SnS 2 -GS 作为锂离子电池 (LIB) 的负极材料表现出优异的容量和循环性能,在 0.1 A g -1(100 次循环)下保持 2007.4 mAh g -1的比容量和 3224.3 0.5 A g -1时的mAh g -1(600 次循环)。MoS 2 /SnS 2 -GS材料的制备方法为二硫化物锂离子电池负极材料的开发提供了很好的参考。

































 京公网安备 11010802027423号
京公网安备 11010802027423号