当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study on the Role of Zn(II) Z-Type Ligands in Facilitating Diaryl Reductive Elimination from Pt(II)
Organometallics ( IF 2.5 ) Pub Date : 2022-12-21 , DOI: 10.1021/acs.organomet.2c00305
Chisondi S. Warioba 1 , Logan G. Jackson 1 , Marliss A. Neal 1 , Brandon E. Haines 1
Organometallics ( IF 2.5 ) Pub Date : 2022-12-21 , DOI: 10.1021/acs.organomet.2c00305
Chisondi S. Warioba 1 , Logan G. Jackson 1 , Marliss A. Neal 1 , Brandon E. Haines 1
Affiliation
|
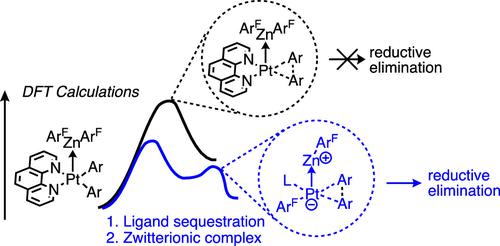
Electron-accepting, or Z-type, ligands offer new potential for affecting the reactivity of transition-metal complexes. A better understanding of the precise mechanisms by which Z-type ligands affect reactivity will aid in taking advantage of their unique properties. It was previously shown that a ZnArF2 additive (where ArF = C6F5) can bind to phenPt(II)Ar2 (where phen = phenanthroline and Ar = p-C6H4tBu) as a Z-type ligand and enable quantitative reductive elimination at 60°C [Liberman-Martin, A. L. Chem. Commun. 2016, 52(43), 7039−7042]. In the present study, density functional theory (DFT) calculations were used to gain insight into the role of the ZnArF2 Z-type ligand in facilitating reductive elimination. The computed Gibbs activation energies with and without ZnArF2 are 41.4 and 44.5 kcal mol–1, respectively. Therefore, simply the presence of the Z-type ligand lowers the barrier by 3.1 kcal mol–1, which is insufficient to account for the experimentally observed effect of ZnArF2. An alternative mechanism is therefore identified. First, the phenanthroline is sequestered by excess ZnArF2 followed by the transfer of one of the ArF groups to the Pt(II) center to form a zwitterionic [ZnArF]+[ArFPtAr2]− complex. An estimate of the overall Gibbs activation energy for this process is 25.1 kcal mol–1, and subsequent reductive elimination occurs with a Gibbs activation energy of 10.1 kcal mol–1. These values are consistent with experimental observations of the reaction. An analysis of the interaction energies of each of the ligands in the reductive elimination model systems generated by the mechanistic study suggests that the effective charge transfer to the Z-type ligand in the zwitterionic complex helps lower the reductive elimination barrier, but the ability to obtain a three-coordinate complex (e.g., in the absence of phenanthroline) is more important.
中文翻译:

Zn(II) Z 型配体促进 Pt(II) 二芳基还原消除作用的计算研究
电子接受或 Z 型配体为影响过渡金属配合物的反应性提供了新的潜力。更好地了解 Z 型配体影响反应性的精确机制将有助于利用其独特的特性。先前表明,ZnAr F 2添加剂(其中Ar F = C 6 F 5)可以作为Z-型配体并在 60°C 下进行定量还原消除 [利伯曼-马丁,阿拉巴马州 化学。公社。 2016 , 52 (43), 7039−7042]。在本研究中,密度泛函理论 (DFT) 计算用于深入了解 ZnAr F 2 Z 型配体在促进还原消除中的作用。使用和不使用 ZnAr F 2时计算出的吉布斯活化能分别为 41.4 和 44.5 kcal mol –1。因此,仅仅 Z 型配体的存在就将势垒降低了 3.1 kcal mol –1,这不足以解释实验观察到的 ZnAr F 2的影响。因此确定了一种替代机制。首先,菲咯啉被过量的 ZnAr F 2螯合随后将其中一个 Ar F基团转移到 Pt(II) 中心,形成两性离子 [ZnAr F ] + [Ar F PtAr 2 ] -络合物。该过程的总吉布斯活化能估计为 25.1 kcal mol –1,随后发生还原消除,吉布斯活化能为 10.1 kcal mol –1. 这些值与反应的实验观察一致。对由机理研究产生的还原消除模型系统中每个配体的相互作用能的分析表明,有效的电荷转移到两性离子复合物中的 Z 型配体有助于降低还原消除屏障,但能够获得三配位络合物(例如,在菲咯啉不存在的情况下)更为重要。
更新日期:2022-12-21
中文翻译:

Zn(II) Z 型配体促进 Pt(II) 二芳基还原消除作用的计算研究
电子接受或 Z 型配体为影响过渡金属配合物的反应性提供了新的潜力。更好地了解 Z 型配体影响反应性的精确机制将有助于利用其独特的特性。先前表明,ZnAr F 2添加剂(其中Ar F = C 6 F 5)可以作为Z-型配体并在 60°C 下进行定量还原消除 [


































 京公网安备 11010802027423号
京公网安备 11010802027423号