当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Holo Structure and Steady State Kinetics of the Thiazolinyl Imine Reductases for Siderophore Biosynthesis
Biochemistry ( IF 2.9 ) Pub Date : 2016-09-15 00:00:00 , DOI: 10.1021/acs.biochem.6b00735
Kathleen M. Meneely 1 , Trey A. Ronnebaum 1 , Andrew P. Riley 1 , Thomas E. Prisinzano 1 , Audrey L. Lamb 1
Biochemistry ( IF 2.9 ) Pub Date : 2016-09-15 00:00:00 , DOI: 10.1021/acs.biochem.6b00735
Kathleen M. Meneely 1 , Trey A. Ronnebaum 1 , Andrew P. Riley 1 , Thomas E. Prisinzano 1 , Audrey L. Lamb 1
Affiliation
|
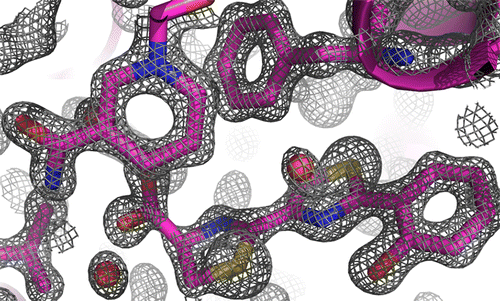
Thiazolinyl imine reductases catalyze the NADPH-dependent reduction of a thiazoline to a thiazolidine, a required step in the formation of the siderophores yersiniabactin (Yersinia spp.) and pyochelin (Pseudomonas aeruginosa). These stand-alone nonribosomal peptide tailoring domains are structural homologues of sugar oxidoreductases. Two closed structures of the thiazolinyl imine reductase from Yersinia enterocolitica (Irp3) are presented here: an NADP+-bound structure to 1.45 Å resolution and a holo structure to 1.28 Å resolution with NADP+ and a substrate analogue bound. Michaelis–Menten kinetics were measured using the same substrate analogue and the homologue from P. aeruginosa, PchG. The data presented here support the hypothesis that tyrosine 128 is the likely general acid residue for catalysis and also highlight the phosphopantetheine tunnel for tethering of the substrate to the nonribosomal peptide synthetase module during assembly line biosynthesis of the siderophore.
中文翻译:

硫唑啉基亚胺还原酶的铁结构和稳态动力学用于铁载体的生物合成
噻唑啉亚胺还原酶催化NADPH依赖性的噻唑啉还原为噻唑烷,这是铁载体耶尔森菌素(Yersinia spp。)和Pyochelin(铜绿假单胞菌(Pseudomonas aeruginosa))形成的必要步骤。这些独立的非核糖体肽修饰域是糖氧化还原酶的结构同源物。此处显示了来自小肠结肠炎耶尔森氏菌(Irp3)的噻唑啉亚胺还原酶的两个封闭结构:NADP + -结合结构(分辨率为1.45Å)和完整结构(分辨率为1.28Å),并带有NADP +和底物类似物。使用相同的底物类似物和铜绿假单胞菌的同系物测量了米氏菌的动力学,PchG。此处提供的数据支持以下假设:酪氨酸128是可能的一般催化酸残基,并且突出显示了在泛铁的组装线生物合成过程中将底物束缚至非核糖体肽合成酶模块的磷酸泛酸通道。
更新日期:2016-09-15
中文翻译:

硫唑啉基亚胺还原酶的铁结构和稳态动力学用于铁载体的生物合成
噻唑啉亚胺还原酶催化NADPH依赖性的噻唑啉还原为噻唑烷,这是铁载体耶尔森菌素(Yersinia spp。)和Pyochelin(铜绿假单胞菌(Pseudomonas aeruginosa))形成的必要步骤。这些独立的非核糖体肽修饰域是糖氧化还原酶的结构同源物。此处显示了来自小肠结肠炎耶尔森氏菌(Irp3)的噻唑啉亚胺还原酶的两个封闭结构:NADP + -结合结构(分辨率为1.45Å)和完整结构(分辨率为1.28Å),并带有NADP +和底物类似物。使用相同的底物类似物和铜绿假单胞菌的同系物测量了米氏菌的动力学,PchG。此处提供的数据支持以下假设:酪氨酸128是可能的一般催化酸残基,并且突出显示了在泛铁的组装线生物合成过程中将底物束缚至非核糖体肽合成酶模块的磷酸泛酸通道。



































 京公网安备 11010802027423号
京公网安备 11010802027423号