Separation and Purification Technology ( IF 8.1 ) Pub Date : 2022-12-21 , DOI: 10.1016/j.seppur.2022.122986 Yang Chen , Zhihui Qu , Hui Hu , Yuanhong Gao
|
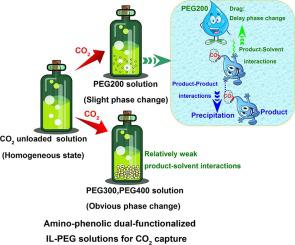
In order to improve the recycling capacity, regenerability and energy efficiency of ionic liquid (IL) based CO2 capture technology, a series of nonaqueous phase change solvents comprising amino-phenolic dual-functionalized ILs and polyethylene glycols (PEGs) were proposed for CO2 capture in this research. The capture performance results demonstrated that the 20 wt% diethylenetriamine-2-bromophenolate(D2Br)-PEG200 and diethylenetriamine-4-bromophenolate(D4Br)-PEG200 solutions with slight phase change could realize high CO2 loading of 1.923 and 1.859 mol/mol IL and CO2-rich phase constituted volume fractions lower than 70%. The absorption ability kept stable at relatively high temperature and the two systems could be rapidly regenerated at 363 K with cyclic regeneration rate above 85% after ten consecutive cycles. The weak thermal stability of the absorption products was proved by TG-DSC analysis, contributing to improved regeneration performance. ATR-IR and 13C NMR analysis confirmed that the amino groups of IL reacted with CO2 to form carbamates, and phenol groups through 1:1 mechanism to form alkyl carbonates, guaranteeing enhanced recycling capacity and regenerability. Spectroscopic analysis and theoretical calculation results revealed the different phase change behaviors presented in PEGs solutions were mainly derived from the product-solvent interaction, which was more influenced by hydroxyl content. The interactions between the product ion pairs could be the main cause of the unstable state of the solutions and phase separation. The different patterns of hydrogen bonding established between IL and PEG molecules could maintain a relatively strong product-solvent interaction in the reacted solution, which would partially overcome the interactions between protonated amine and carbamate, thus delaying the phase transition formation and forming a slight solid–liquid phase change, potentially beneficial for CO2 capture. The two systems experienced a low reaction heat of 1.315 and 0.782 GJ/t CO2, which could significantly reduce regeneration heat load. Therefore, the amino-phenolic dual-functionalized IL solutions are proposed to be a competitive and reversible CO2 trapping agent.
中文翻译:

用于可逆 CO2 捕获的非水氨基酚双功能化离子液体吸收剂:相变行为和机制
为了提高基于离子液体 (IL) 的 CO 2捕获技术的回收能力、再生性和能源效率,提出了一系列包含氨基酚双功能化 IL 和聚乙二醇 (PEG) 的非水相变溶剂用于 CO 2在这项研究中捕获。捕获性能结果表明,具有轻微相变的 20 wt% 二亚乙基三胺-2-溴苯酚 (D2Br)-PEG200 和二亚乙基三胺-4-溴苯酚 (D4Br)-PEG200 溶液可实现1.923 和 1.859 mol/mol IL 的高 CO 2负载量和CO 2-富相构成的体积分数低于 70%。吸收能力在较高温度下保持稳定,两个体系可在363 K快速再生,连续十次循环后循环再生率在85%以上。TG-DSC分析证明吸收产物的热稳定性较弱,有助于提高再生性能。ATR-IR和13 C NMR分析证实IL的氨基与CO 2反应形成氨基甲酸酯,和酚基通过1:1的机制形成碳酸烷基酯,保证增强的回收能力和再生能力。光谱分析和理论计算结果表明,PEGs溶液中呈现的不同相变行为主要来源于产物-溶剂相互作用,而羟基含量对这种相互作用的影响更大。产物离子对之间的相互作用可能是溶液不稳定状态和相分离的主要原因。IL和PEG分子之间建立的不同氢键模式可以在反应溶液中保持相对强的产物-溶剂相互作用,这将部分克服质子化胺和氨基甲酸酯之间的相互作用,2捕获。这两个系统经历了 1.315 和 0.782 GJ/t CO 2的低反应热,这可以显着降低再生热负荷。因此,氨基酚双官能化离子液体溶液被认为是一种具有竞争力的可逆CO 2捕集剂。





























 京公网安备 11010802027423号
京公网安备 11010802027423号