当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photolysis versus Photothermolysis of N2O on a Semiconductor Surface Revealed by Nonadiabatic Molecular Dynamics
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-21 , DOI: 10.1021/jacs.2c10643 Cheng Cheng 1 , Oleg V Prezhdo 2 , Run Long 1 , Wei-Hai Fang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-21 , DOI: 10.1021/jacs.2c10643 Cheng Cheng 1 , Oleg V Prezhdo 2 , Run Long 1 , Wei-Hai Fang 1
Affiliation
|
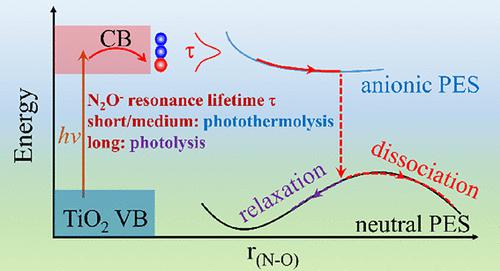
Identifying photolysis and photothermolysis during a photochemical reaction has remained challenging because of the highly non-equilibrium and ultrafast nature of the processes. Using state-of-the-art ab initio adiabatic and nonadiabatic molecular dynamics, we investigate N2O photodissociation on the reduced rutile TiO2(110) surface and establish its detailed mechanism. The photodecomposition is initiated by electron injection, leading to the formation of a N2O– ion-radical, and activation of the N2O bending and symmetric stretching vibrations. Photothermolysis governs the N2O dissociation when N2O– is short-lived. The dissociation is activated by a combination of the anionic excited state evolution and local heating. A thermal fluctuation drives the molecular acceptor level below the TiO2 band edge, stabilizes the N2O– anion radical, and causes dissociation on a 1 ps timescale. As the N2O– resonance lifetime increases, photolysis becomes dominant since evolution in the anionic excited state activates the bending and symmetric stretching of N2O, inducing the dissociation. The photodecomposition occurs more easily when N2O is bonded to TiO2 through the O rather than N atom. We demonstrate further that a thermal dissociation of N2O can be realized by a rational choice of metal dopants, which enhance p–d orbital hybridization, facilitate electron transfer, and break N2O spontaneously. By investigating the charge dynamics and lifetime, we provide a fundamental atomistic understanding of the competition and synergy between the photocatalytic and photothermocatalytic dissociation of N2O and demonstrate how N2O reduction can be controlled by light irradiation, adsorption configuration, and dopants, enabling the design of high-performance transition-metal oxide catalysts.
中文翻译:

非绝热分子动力学揭示的半导体表面上 N2O 的光解与光热解
由于过程的高度非平衡和超快性质,在光化学反应过程中识别光解和光热解仍然具有挑战性。使用最先进的从头算绝热和非绝热分子动力学,我们研究了还原金红石 TiO 2 (110) 表面上的 N 2 O 光解离并建立了其详细机制。光分解由电子注入引发,导致形成 N 2 O -离子自由基,并激活 N 2 O 弯曲和对称伸缩振动。当 N 2 O –时,光热分解控制 N 2 O 解离是短暂的。解离由阴离子激发态演变和局部加热的组合激活。热波动驱动分子受体水平低于 TiO 2带边缘,稳定 N 2 O -阴离子自由基,并导致 1 ps 时间尺度的解离。随着 N 2 O -共振寿命的增加,光解成为主导,因为阴离子激发态的演化激活了 N 2 O 的弯曲和对称拉伸,从而诱导解离。当N 2 O与TiO 2键合时更容易发生光分解通过 O 而不是 N 原子。我们进一步证明,通过合理选择金属掺杂剂可以实现N 2 O 的热解离,从而增强p – d轨道杂化,促进电子转移,并自发地分解 N 2 O。通过研究电荷动力学和寿命,我们提供了对 N 2 O的光催化和光热催化解离之间的竞争和协同作用的基本原子理解,并展示了如何通过光照射、吸附配置和掺杂剂控制N 2 O 还原,从而实现高性能过渡金属氧化物催化剂的设计。
更新日期:2022-12-21
中文翻译:

非绝热分子动力学揭示的半导体表面上 N2O 的光解与光热解
由于过程的高度非平衡和超快性质,在光化学反应过程中识别光解和光热解仍然具有挑战性。使用最先进的从头算绝热和非绝热分子动力学,我们研究了还原金红石 TiO 2 (110) 表面上的 N 2 O 光解离并建立了其详细机制。光分解由电子注入引发,导致形成 N 2 O -离子自由基,并激活 N 2 O 弯曲和对称伸缩振动。当 N 2 O –时,光热分解控制 N 2 O 解离是短暂的。解离由阴离子激发态演变和局部加热的组合激活。热波动驱动分子受体水平低于 TiO 2带边缘,稳定 N 2 O -阴离子自由基,并导致 1 ps 时间尺度的解离。随着 N 2 O -共振寿命的增加,光解成为主导,因为阴离子激发态的演化激活了 N 2 O 的弯曲和对称拉伸,从而诱导解离。当N 2 O与TiO 2键合时更容易发生光分解通过 O 而不是 N 原子。我们进一步证明,通过合理选择金属掺杂剂可以实现N 2 O 的热解离,从而增强p – d轨道杂化,促进电子转移,并自发地分解 N 2 O。通过研究电荷动力学和寿命,我们提供了对 N 2 O的光催化和光热催化解离之间的竞争和协同作用的基本原子理解,并展示了如何通过光照射、吸附配置和掺杂剂控制N 2 O 还原,从而实现高性能过渡金属氧化物催化剂的设计。















































 京公网安备 11010802027423号
京公网安备 11010802027423号