当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rylene and Rylene Diimides: Comparison of Theoretical and Experimental Results and Prediction for High-Rylene Derivatives
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2016-09-15 00:00:00 , DOI: 10.1021/acs.jpca.6b07552 Xiaohong Zhao 1 , Yushuai Xiong 1 , Jie Ma 1 , Zhongyi Yuan 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2016-09-15 00:00:00 , DOI: 10.1021/acs.jpca.6b07552 Xiaohong Zhao 1 , Yushuai Xiong 1 , Jie Ma 1 , Zhongyi Yuan 1
Affiliation
|
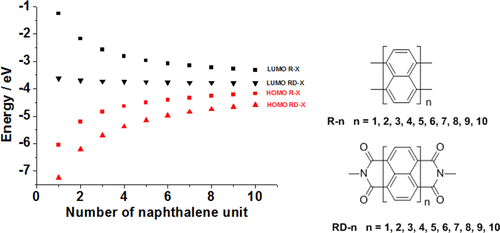
Low rylene (R) and rylene diimides (RD) are important organic semiconductors and dyes. High R and RD with larger conjugated cores show different properties compared with their low counterparts. Herein, absorption spectra, frontier molecular orbitals, band gaps, inner-sphere reorganization energy (λi), ionization potential, electron affinity, and atomic charge population of 20 rylene compounds were calculated by the density functional theory method. The theoretical results agree well with experimental ones. We predict some unusual properties of some high rylene derivatives that are unknown compounds due to synthetic difficulties. The lowest unoccupied molecular orbital energy levels of RD compounds change slightly, from −3.61 to −3.79 eV, which makes them strong electron acceptors. The band gaps narrow with the size increase of conjugated cores, which makes high rylene derivatives near-infrared dyes. The rising highest occupied molecular orbital energy levels of high rylene derivatives makes them unstable in the air. The λi falls with the size increase of the conjugated core, and the size of RD-4 or R-4 is big enough for the small λi to favor charge transport. The charge population analysis indicates R and RD have different charge distribution under the effect of electron-withdrawing imide groups, which contributes to distinct properties.
中文翻译:

Rylene和Rylene二酰亚胺:理论和实验结果的比较以及对高Rylene衍生物的预测
低萘嵌苯(R)和萘嵌二酰亚胺(RD)是重要的有机半导体和染料。具有较大共轭磁芯的高R和RD与低共轭磁芯相比具有不同的性能。在此,吸收光谱,前沿分子轨道,带隙,内球重组能(λ我),通过密度泛函理论方法计算了20种萘嵌苯化合物的电离势,电子亲和力和原子电荷总数。理论结果与实验结果吻合良好。由于合成困难,我们预测了一些未知化合物的高芳纶衍生物的某些非常规性质。RD化合物的最低未占据分子轨道能级稍有变化,从-3.61到-3.79 eV,这使它们成为强电子受体。带隙随着共轭核尺寸的增加而变窄,这使得高芳纶衍生物成为近红外染料。高芳纶衍生物的最高占据分子轨道能级的上升使得它们在空气中不稳定。该λ我与共轭核心的尺寸增加,并且RD-4或R-4的尺寸落在足够大的小λ我有利于电荷传输。电荷总体分析表明,R和RD在吸电子酰亚胺基团的作用下具有不同的电荷分布,这有助于形成不同的性质。
更新日期:2016-09-15
中文翻译:

Rylene和Rylene二酰亚胺:理论和实验结果的比较以及对高Rylene衍生物的预测
低萘嵌苯(R)和萘嵌二酰亚胺(RD)是重要的有机半导体和染料。具有较大共轭磁芯的高R和RD与低共轭磁芯相比具有不同的性能。在此,吸收光谱,前沿分子轨道,带隙,内球重组能(λ我),通过密度泛函理论方法计算了20种萘嵌苯化合物的电离势,电子亲和力和原子电荷总数。理论结果与实验结果吻合良好。由于合成困难,我们预测了一些未知化合物的高芳纶衍生物的某些非常规性质。RD化合物的最低未占据分子轨道能级稍有变化,从-3.61到-3.79 eV,这使它们成为强电子受体。带隙随着共轭核尺寸的增加而变窄,这使得高芳纶衍生物成为近红外染料。高芳纶衍生物的最高占据分子轨道能级的上升使得它们在空气中不稳定。该λ我与共轭核心的尺寸增加,并且RD-4或R-4的尺寸落在足够大的小λ我有利于电荷传输。电荷总体分析表明,R和RD在吸电子酰亚胺基团的作用下具有不同的电荷分布,这有助于形成不同的性质。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号