Synthesis ( IF 2.2 ) Pub Date : 2022-12-20 , DOI: 10.1055/a-1983-2140 Xavier Salom-Roig 1 , Arie van der Lee 2 , Erwann Grenet 3
|
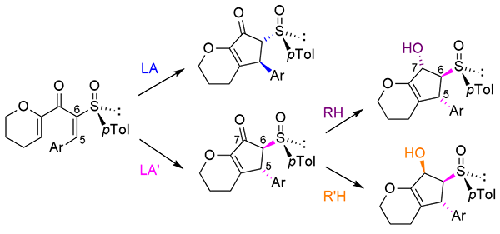
Activated dienones bearing a chiral sulfoxide were transformed diastereodivergently into the corresponding disubstituted 3,4,5,6-tetrahydrocyclopenta[b]pyran-7(2H)-ones. The torquoselectivity of the reaction could be switched by changing the Lewis acid used as promoter. From the four possible stereoisomers, only the two trans were observed. In a second switchable diastereodivergent step, both corresponding diastereomeric 2,3,4,5,6,7-hexahydrocyclopenta[b]pyran-7-ols were obtained via the diastereoselective reduction of the ketone by changing the reducing agent. The Lewis acids as well as the reducing agents employed in both diastereodivergent steps were achiral, the diastereoselectivities being dictated by the sulfinyl auxiliary.
中文翻译:

由手性亚砜介导的双开关非对映发散策略通过纳扎罗夫环化获得双取代四氢环戊吡喃酮和六氢环戊吡喃醇支架
带有手性亚砜的活化二烯酮被非对映发散地转化为相应的双取代 3,4,5,6-四氢环戊[ b ]吡喃-7(2 H )-酮。可以通过改变用作促进剂的路易斯酸来切换反应的扭矩选择性。在四种可能的立体异构体中,仅观察到两种反式。在第二个可切换的非对映发散步骤中,通过改变还原剂对酮进行非对映选择性还原,获得了相应的非对映体 2,3,4,5,6,7-六氢环戊二烯[ b ]吡喃-7-醇。在两个非对映发散步骤中使用的路易斯酸和还原剂都是非手性的,非对映选择性由亚磺酰基助剂决定。















































 京公网安备 11010802027423号
京公网安备 11010802027423号