当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Alkoxy Radicals as Hydrogen Atom Transfer Agents in Ce-Catalyzed C–H Functionalization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-20 , DOI: 10.1021/jacs.2c10126 Qing An 1, 2 , Yang-Yang Xing 3, 4, 5 , Ruihua Pu 1 , Menghui Jia 6 , Yuegang Chen 1 , Anhua Hu 7 , Shuo-Qing Zhang 3, 4, 5 , Na Yu 1 , Jianbo Du 1, 2 , Yanxia Zhang 2 , Jinquan Chen 6 , Weimin Liu 1 , Xin Hong 3, 4, 5 , Zhiwei Zuo 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-20 , DOI: 10.1021/jacs.2c10126 Qing An 1, 2 , Yang-Yang Xing 3, 4, 5 , Ruihua Pu 1 , Menghui Jia 6 , Yuegang Chen 1 , Anhua Hu 7 , Shuo-Qing Zhang 3, 4, 5 , Na Yu 1 , Jianbo Du 1, 2 , Yanxia Zhang 2 , Jinquan Chen 6 , Weimin Liu 1 , Xin Hong 3, 4, 5 , Zhiwei Zuo 2
Affiliation
|
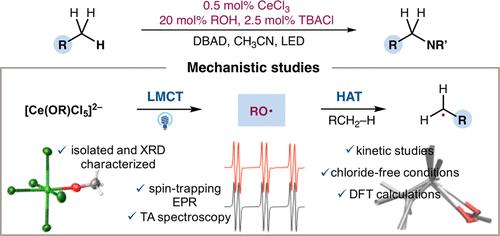
The intermediacy of alkoxy radicals in cerium-catalyzed C–H functionalization via H-atom abstraction has been unambiguously confirmed. Catalytically relevant Ce(IV)–alkoxide complexes have been synthesized and characterized by X-ray diffraction. Operando electron paramagnetic resonance and transient absorption spectroscopy experiments on isolated pentachloro Ce(IV) alkoxides identified alkoxy radicals as the sole heteroatom-centered radical species generated via ligand-to-metal charge transfer (LMCT) excitation. Alkoxy-radical-mediated hydrogen atom transfer (HAT) has been verified via kinetic analysis, density functional theory (DFT) calculations, and reactions under strictly chloride-free conditions. These experimental findings unambiguously establish the critical role of alkoxy radicals in Ce-LMCT catalysis and definitively preclude the involvement of chlorine radical. This study has also reinforced the necessity of a high relative ratio of alcohol vs Ce for the selective alkoxy-radical-mediated HAT, as seemingly trivial changes in the relative ratio of alcohol vs Ce can lead to drastically different mechanistic pathways. Importantly, the previously proposed chlorine radical–alcohol complex, postulated to explain alkoxy-radical-enabled selectivities in this system, has been examined under scrutiny and ruled out by regioselectivity studies, transient absorption experiments, and high-level calculations. Moreover, the peculiar selectivity of alkoxy radical generation in the LMCT homolysis of Ce(IV) heteroleptic complexes has been analyzed and back-electron transfer (BET) may have regulated the efficiency and selectivity for the formation of ligand-centered radicals.
中文翻译:

Ce 催化的 C-H 官能化中作为氢原子转移剂的烷氧基的鉴定
烷氧基自由基在铈催化的 C-H 官能化中通过 H 原子抽取的中介作用已得到明确证实。已经合成了催化相关的 Ce(IV)-醇盐络合物,并通过 X 射线衍射对其进行了表征。分离的五氯 Ce(IV) 醇盐的原位电子顺磁共振和瞬态吸收光谱实验将烷氧基自由基确定为通过配体到金属电荷转移 (LMCT) 激发产生的唯一以杂原子为中心的自由基物种。烷氧基自由基介导的氢原子转移 (HAT) 已通过动力学分析、密度泛函理论 (DFT) 计算和严格无氯条件下的反应得到验证。这些实验结果明确地确立了烷氧基自由基在 Ce-LMCT 催化中的关键作用,并明确排除了氯自由基的参与。这项研究还强化了选择性烷氧基自由基介导的 HAT 中酒精与 Ce 的高相对比例的必要性,因为酒精与 Ce 的相对比例看似微不足道的变化可能导致截然不同的机制途径。重要的是,之前提出的氯自由基-醇络合物假设解释了该系统中烷氧基自由基启用的选择性,已经过仔细检查并通过区域选择性研究、瞬态吸收实验和高级计算排除。而且,
更新日期:2022-12-20
中文翻译:

Ce 催化的 C-H 官能化中作为氢原子转移剂的烷氧基的鉴定
烷氧基自由基在铈催化的 C-H 官能化中通过 H 原子抽取的中介作用已得到明确证实。已经合成了催化相关的 Ce(IV)-醇盐络合物,并通过 X 射线衍射对其进行了表征。分离的五氯 Ce(IV) 醇盐的原位电子顺磁共振和瞬态吸收光谱实验将烷氧基自由基确定为通过配体到金属电荷转移 (LMCT) 激发产生的唯一以杂原子为中心的自由基物种。烷氧基自由基介导的氢原子转移 (HAT) 已通过动力学分析、密度泛函理论 (DFT) 计算和严格无氯条件下的反应得到验证。这些实验结果明确地确立了烷氧基自由基在 Ce-LMCT 催化中的关键作用,并明确排除了氯自由基的参与。这项研究还强化了选择性烷氧基自由基介导的 HAT 中酒精与 Ce 的高相对比例的必要性,因为酒精与 Ce 的相对比例看似微不足道的变化可能导致截然不同的机制途径。重要的是,之前提出的氯自由基-醇络合物假设解释了该系统中烷氧基自由基启用的选择性,已经过仔细检查并通过区域选择性研究、瞬态吸收实验和高级计算排除。而且,















































 京公网安备 11010802027423号
京公网安备 11010802027423号