Separation and Purification Technology ( IF 8.1 ) Pub Date : 2022-12-13 , DOI: 10.1016/j.seppur.2022.122886 Xue-Li Chen , Haitao Li , LanHai Lai , YueXing Zhang , Yonglin Chen , XiaoKang Li , Bin Liu , HuiJuan Wang
|
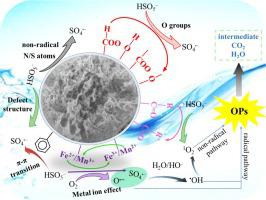
Exploiting stable and high-performance catalysts is a challenge in remediating organic pollutants (OPs) during advanced oxidation. Herein, this study reported MnFe2O4 decorated biochar (MnFe2O4/BC) as an adsorptive-catalyst for peroxymonosulfate (PMS) activation to degrade OPs. BC as support not only increased the stability and dispersibility but also decreased the particle diameter of MnFe2O4. We demonstrated various OPs (50 mL, 20 mg·L−1) (including malachite green, bisphenol A, methylene blue, sulfamethoxazole, tetracycline, and thiacloprid) was synergistically adsorbed and oxidized within 10 min with the introduction of PMS (0.65 mM) in the MnFe2O4/BC system. The degradation efficiency was more than 95% after recycling six times. Results of discrete Fourier transform revealed that PMS was preferentially adsorbed on BC doping sites (−4.31 eV to −3.85 eV) and MnFe2O4 parts (−9.67 eV), and then the adsorbed–PMS was activated. These results confirmed that oxidation occurs through radical–induced and non–radical pathways in the MnFe2O4/BC system. Overall, the MnFe2O4/BC showed efficient performance, also this work provides a new insight for understanding of the PMS activation mechanism.
中文翻译:

使用 MnFe2O4 改性生物炭活化过氧单硫酸盐降解有机污染物:性能和机制
开发稳定和高性能的催化剂是在高级氧化过程中修复有机污染物 (OPs) 的挑战。在此,本研究报道了 MnFe 2 O 4修饰的生物炭 (MnFe 2 O 4 /BC) 作为过氧单硫酸盐 (PMS) 活化的吸附催化剂以降解 OPs。BC作为载体不仅提高了稳定性和分散性,而且降低了MnFe 2 O 4的粒径。我们证明了各种 OPs(50 mL,20 mg·L -1)(包括孔雀石绿、双酚 A、亚甲蓝、磺胺甲恶唑、四环素和噻虫啉)在引入 PMS (0.65 mM) 后 10 分钟内被协同吸附和氧化在锰铁2 O 4 /BC体系。循环使用6次后降解效率在95%以上。离散傅里叶变换的结果表明,PMS 优先吸附在 BC 掺杂位点(-4.31 eV 至 -3.85 eV)和 MnFe 2 O 4部分(-9.67 eV),然后吸附的-PMS 被激活。这些结果证实,氧化通过 MnFe 2 O 4 /BC 系统中的自由基诱导和非自由基途径发生。总体而言,MnFe 2 O 4 /BC 表现出高效的性能,这项工作也为理解 PMS 激活机制提供了新的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号