Chemosphere ( IF 8.1 ) Pub Date : 2022-12-14 , DOI: 10.1016/j.chemosphere.2022.137520 Yangyang Bai 1 , Xiaoqin Sun 1 , Yuan Dang 1 , Sha Yu 1 , Jun-Jie Zhu 2 , Yuanzhen Zhou 1
|
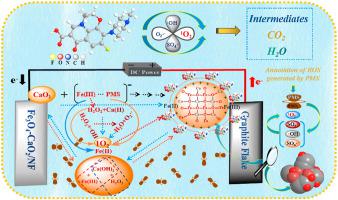
Electro-Fenton reaction was limited by the generation of H2O2 and the circulation of Fe(Ⅱ)/Fe(Ⅲ). Herein, an efficient electro-Fenton-like process was constructed based on Fe3O4–CaO2 cathode promoted by peroxymonosulfate (PMS). Levofloxacin (LEV) could be efficiently degraded (92.1%) and mineralized with the TOC removal of 74.5% in this self-circulating process. More importantly, the Fe3O4–CaO2 exhibited good stability in the recycles due that CaO2 was covered by Fe3O4, which inhibited the rapid release of H2O2. Mechanism analysis indicated that CaO2 could not only replace H2O2 to accelerate the oxidation of Fe(Ⅱ) to Fe(Ⅲ), but also could form complexes with Fe(Ⅲ) and PMS to transfer electrons from ligands to metals, thereby enhancing the reduction of Fe(Ⅲ) to Fe(Ⅱ). As a result, the electrical consumption was significantly reduced, which was only 5.0% of the Fe3O4 in electro-Fenton reaction. Meanwhile, the hydrolyzed product of Ca(OH)2 reacted with Fe(Ⅲ) in the presence of H2O2 and converted into CaO2. Thus, the self-circulation of CaO2/Ca(OH)2 and Fe(Ⅲ)/Fe(Ⅱ) was realized, which accelerated the generation of active species, such as, ·OH, O2·- and 1O2. This work first proposed a self-circulating electro-Fenton-like system and demonstrated the potential application of Fe3O4–CaO2 in the treatment of wastewater.
中文翻译:

Fe3O4-CaO2 阴极上的自循环类电子芬顿过程高效降解左氧氟沙星
Electro-Fenton反应受到H 2 O 2的生成和Fe(Ⅱ)/Fe(Ⅲ)循环的限制。在此,基于由过氧单硫酸盐 (PMS) 促进的Fe 3 O 4 –CaO 2阴极构建了一种高效的类芬顿过程。在这个自循环过程中,左氧氟沙星 (LEV) 可以有效降解 (92.1%) 并矿化,TOC 去除率为 74.5%。更重要的是,Fe 3 O 4 –CaO 2在循环中表现出良好的稳定性,因为 CaO 2被 Fe 3 O 4覆盖,抑制了 H 2 O 2的快速释放. 机理分析表明,CaO 2不仅可以代替H 2 O 2加速Fe(Ⅱ)氧化为Fe(Ⅲ),还可以与Fe(Ⅲ)和PMS形成络合物,将电子从配体转移到金属,从而促进Fe(Ⅲ)向Fe(Ⅱ)的还原。结果,电耗显着降低,仅为电-Fenton反应中Fe 3 O 4的5.0%。同时,Ca(OH) 2的水解产物在H 2 O 2存在下与Fe(Ⅲ)反应并转化为CaO 2。因此,CaO 2 /Ca(OH) 2的自循环实现了Fe(Ⅲ)/Fe(Ⅱ),加速了·OH、O 2 · -和1 O 2等活性物质的生成。这项工作首先提出了一种自循环类电芬顿系统,并展示了Fe 3 O 4 –CaO 2在废水处理中的潜在应用。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号