Chem Catalysis ( IF 11.5 ) Pub Date : 2022-12-13 , DOI: 10.1016/j.checat.2022.11.014 Seunghwa Lee , You-Chiuan Chu , Lichen Bai , Hao Ming Chen , Xile Hu
|
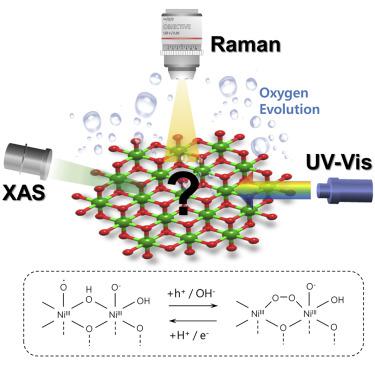
The oxygen evolution reaction (OER) is a key electrochemical reaction relevant to energy storage. Ni-containing bimetallic oxyhydroxides are among the most active OER catalysts in alkaline medium, but the mechanism of OER on pure Ni oxyhydroxide remains unclear. Here we combine multiple operando spectroscopic tools including X-ray absorption, ultraviolet visible (UV-Vis), and Raman with electrokinetics to study the mechanism of OER on Ni(OH)2 nanosheets. The spectroscopic data reveal two intermediates. The first one is a Ni(III)-O⋅ species formed upon a 2-e oxidation of Ni(OH)2, and the second one is a Ni-OO-Ni species formed upon a 1-e oxidation of Ni(III)-O⋅. The Ni-OO-Ni species is a side-on superoxide that acts as a site for hole accumulation. The reaction kinetics follows an inner-sphere model. The rate-determining step is OH− attack to Ni(III)-O⋅, chemically driven by the Ni-OO-Ni species. This work provides new experimental fingerprints and mechanistic perspectives for the understanding of Ni-based OER catalysts.
中文翻译:

侧接镍超氧化物中间体的原位鉴定和羟基氧化镍的析氧机制
析氧反应 (OER) 是与储能相关的关键电化学反应。含镍双金属羟基氧化物是碱性介质中最活跃的 OER 催化剂之一,但纯镍羟基氧化物的 OER 机理仍不清楚。在这里,我们将包括 X 射线吸收、紫外可见 (UV-Vis) 和拉曼在内的多种原位光谱工具与电动学相结合,以研究 OER 在 Ni(OH) 2纳米片上的机理。光谱数据揭示了两个中间体。第一个是 Ni(III)-O⋅ 物种在 Ni(OH) 2的 2-e 氧化时形成,第二个是在 Ni(III)-O⋅ 的 1-e 氧化时形成的 Ni-OO-Ni 物种。Ni-OO-Ni 物种是一种侧面超氧化物,可作为空穴积累的场所。反应动力学遵循内球模型。决速步骤是 OH −攻击 Ni(III)-O⋅,由 Ni-OO-Ni 物种化学驱动。这项工作为理解镍基 OER 催化剂提供了新的实验指纹和机理观点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号