当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Intermediates in Artificial Photosynthesis with Molecular Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-12-15 , DOI: 10.1021/acscatal.2c05033
Young Hyun Hong 1 , Yong-Min Lee 1 , Wonwoo Nam 1 , Shunichi Fukuzumi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-12-15 , DOI: 10.1021/acscatal.2c05033
Young Hyun Hong 1 , Yong-Min Lee 1 , Wonwoo Nam 1 , Shunichi Fukuzumi 1
Affiliation
|
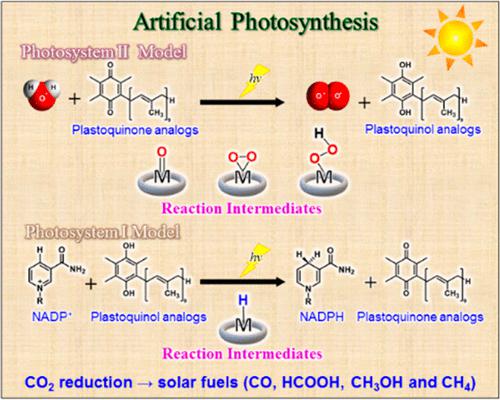
In nature, water oxidation is catalyzed by Mn4Ca clusters in the oxygen-evolving complex (OEC) of photosystem II (PSII), in which a manganese(V)-oxo species acts as an active reaction intermediate. Electrons and protons taken from water in PSII are used to reduce plastoquinone to plastoquinol via photoinduced charge separation in the photosynthetic reaction center. In photosystem I (PSI), NADP+ coenzyme is reduced by plastoquinol via photoinduced charge separation in the photosynthetic reaction center to produce NADPH, which is used as a reductant to reduce CO2 to carbohydrates in the Calvin cycle. Extensive efforts have so far been made to mimic functions of PSII and PSI for photocatalytic water oxidation and reduction to produce O2 and H2, respectively. Characterization and reactivity of high-valent metal-oxo, -hydroperoxo, -peroxo, and -superoxo intermediates have been investigated to clarify the mechanisms of water oxidation. Metal hydride complexes have also been studied in relation with the catalytic reactivity for water reduction to produce H2 as well as NAD+ reduction to NADH. This Review is intended to provide an overview on the functional model reactions of PSII and PSI for the photocatalytic water oxidation and reduction, respectively. The roles of high-valent metal-oxo, -hydroperoxo, -peroxo, and -superoxo complexes as the reaction intermediates in photocatalytic water oxidation are focused in relation with the catalytic mechanisms of water oxidation. The roles of metal hydride complexes are also discussed in relation with the catalytic mechanisms of hydrogen evolution and NAD+ reduction to NADH. The combination of functional model reactions of PSII and PSI leads to construct molecular artificial photosynthetic systems in which water is split to H2 and O2 in a 2:1 ratio, providing a way to realize artificial photosynthesis in molecular levels.
中文翻译:

分子催化剂人工光合作用中的反应中间体
在自然界中,水氧化由光系统 II (PSII) 的放氧复合物 (OEC) 中的Mn 4 Ca 簇催化,其中锰 (V)-氧代物质充当活性反应中间体。PSII 中从水中获取的电子和质子用于通过光合反应中心的光诱导电荷分离将质体醌还原为质体醌。在光系统 I (PSI) 中,NADP +辅酶在光合反应中心通过光诱导电荷分离被质体醌还原,生成 NADPH,在卡尔文循环中用作还原剂将 CO 2还原为碳水化合物。迄今为止,已经做出广泛的努力来模拟 PSII 和 PSI 的功能,用于光催化水氧化和还原以产生 O 2和H 2,分别。研究了高价金属-氧代、-氢过氧代、-过氧代和-超氧代中间体的表征和反应性,以阐明水氧化的机制。还研究了金属氢化物络合物与水还原产生 H 2以及 NAD +的催化反应性的关系还原为 NADH。本综述旨在概述 PSII 和 PSI 分别用于光催化水氧化和还原的功能模型反应。高价金属-oxo、-hydroperoxo、-peroxo 和-superoxo 配合物作为光催化水氧化反应中间体的作用与水氧化的催化机制有关。金属氢化物络合物的作用还与析氢和 NAD +还原为 NADH 的催化机制相关进行了讨论。结合PSII和PSI的功能模型反应,构建分子人工光合系统,其中水分解为H 2和O 2以2:1的比例,提供了一种在分子水平上实现人工光合作用的途径。
更新日期:2022-12-15
中文翻译:

分子催化剂人工光合作用中的反应中间体
在自然界中,水氧化由光系统 II (PSII) 的放氧复合物 (OEC) 中的Mn 4 Ca 簇催化,其中锰 (V)-氧代物质充当活性反应中间体。PSII 中从水中获取的电子和质子用于通过光合反应中心的光诱导电荷分离将质体醌还原为质体醌。在光系统 I (PSI) 中,NADP +辅酶在光合反应中心通过光诱导电荷分离被质体醌还原,生成 NADPH,在卡尔文循环中用作还原剂将 CO 2还原为碳水化合物。迄今为止,已经做出广泛的努力来模拟 PSII 和 PSI 的功能,用于光催化水氧化和还原以产生 O 2和H 2,分别。研究了高价金属-氧代、-氢过氧代、-过氧代和-超氧代中间体的表征和反应性,以阐明水氧化的机制。还研究了金属氢化物络合物与水还原产生 H 2以及 NAD +的催化反应性的关系还原为 NADH。本综述旨在概述 PSII 和 PSI 分别用于光催化水氧化和还原的功能模型反应。高价金属-oxo、-hydroperoxo、-peroxo 和-superoxo 配合物作为光催化水氧化反应中间体的作用与水氧化的催化机制有关。金属氢化物络合物的作用还与析氢和 NAD +还原为 NADH 的催化机制相关进行了讨论。结合PSII和PSI的功能模型反应,构建分子人工光合系统,其中水分解为H 2和O 2以2:1的比例,提供了一种在分子水平上实现人工光合作用的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号