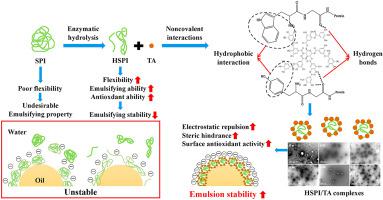
随着人们对健康和可持续饮食模式的认识不断提高,植物蛋白作为动物蛋白的替代品越来越受到人们的关注。同时,蛋白质与天然酚类化合物的非共价相互作用可用于开发功能性成分。在这项工作中,大豆分离蛋白 (HSPI) 的碱性蛋白酶水解物用于制备与单宁酸 (TA) 的复合物,并研究了它们的理化性质,并与大豆分离蛋白 (SPI) 和酪蛋白酸钠 (SC) 进行了比较,以深入了解多酚改性的优势。随着 TA 浓度的增加,复合溶液的粒径减小,浊度升高,表面电荷增加。相互作用主要通过氢键和疏水相互作用发生,导致蛋白质分子结构的展开。随着 TA 浓度的增加,复合物显示出较低的界面吸附活性和降低的界面粘弹性模量,这可能是因为 TA 干扰了蛋白质水解物形成弹性膜的扩散和分子相互作用。尽管如此,基于复合物的乳液显示出增强的乳化稳定性,液滴尺寸小,优异的氧化稳定性,由于较高的表面电荷和颗粒稳定的界面膜的形成而对热和冻融处理具有高物理稳定性。这些发现为开发具有优异乳化性能的植物蛋白提供了见解,显示出在不含乳制品的乳化产品中作为 SC 替代品的巨大应用潜力。随着 TA 浓度的增加,复合物显示出较低的界面吸附活性和降低的界面粘弹性模量,这可能是因为 TA 干扰了蛋白质水解物形成弹性膜的扩散和分子相互作用。尽管如此,基于复合物的乳液显示出增强的乳化稳定性,液滴尺寸小,优异的氧化稳定性,由于较高的表面电荷和颗粒稳定的界面膜的形成而对热和冻融处理具有高物理稳定性。这些发现为开发具有优异乳化性能的植物蛋白提供了见解,显示出在不含乳制品的乳化产品中作为 SC 替代品的巨大应用潜力。随着 TA 浓度的增加,复合物显示出较低的界面吸附活性和降低的界面粘弹性模量,这可能是因为 TA 干扰了蛋白质水解物形成弹性膜的扩散和分子相互作用。尽管如此,基于复合物的乳液显示出增强的乳化稳定性,液滴尺寸小,优异的氧化稳定性,由于较高的表面电荷和颗粒稳定的界面膜的形成而对热和冻融处理具有高物理稳定性。这些发现为开发具有优异乳化性能的植物蛋白提供了见解,显示出在不含乳制品的乳化产品中作为 SC 替代品的巨大应用潜力。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Structural characterization, interfacial and emulsifying properties of soy protein hydrolysate-tannic acid complexes
With increasing awareness of health and sustainable dietary pattern, plant-based proteins have shown a growing interest as substitutes for animal proteins. Meanwhile, the non-covalent interaction of protein with natural phenolic compounds can be used to develop functional ingredients. In this work, Alcalase hydrolysates of soy protein isolate (HSPI) were used to fabricate complexes with tannic acid (TA), and their physicochemical properties were investigated and compared with soy protein isolate (SPI) and sodium caseinate (SC) to get insights into the advantage of polyphenol modification. The complex solution showed reduced particle size, higher turbidity, and higher surface charge with increasing TA concentration. The interaction mainly occurred through hydrogen bond and hydrophobic interaction, leading to the unfolding of protein molecular structure. The complexes displayed lower interfacial adsorption activity and reduced interfacial viscoelastic modulus upon increasing TA concentration possibly because of the interference of TA on the diffusion and molecule interaction of protein hydrolysates to form elastic films. Nonetheless, the complex-based emulsion showed enhanced emulsifying stability with small droplet size, superior oxidation stability, high physical stability against heat and freeze-thaw treatment due to the higher surface charge and formation of particle-stabilized interfacial membrane. These findings provide insights for developing plant-based protein with excellent emulsifying properties, which show great potential for application as SC substitute in dairy-free emulsified products.
































 京公网安备 11010802027423号
京公网安备 11010802027423号