当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C3-Cyanation of Pyridines: Constraints on Electrophiles and Determinants of Regioselectivity
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-12-14 , DOI: 10.1002/anie.202216894 Ming Zhang 1 , Qingyang Zhou 2 , Heng Luo 1 , Zi-Lu Tang 1 , Xiufang Xu 2 , Xiao-Chen Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-12-14 , DOI: 10.1002/anie.202216894 Ming Zhang 1 , Qingyang Zhou 2 , Heng Luo 1 , Zi-Lu Tang 1 , Xiufang Xu 2 , Xiao-Chen Wang 1
Affiliation
|
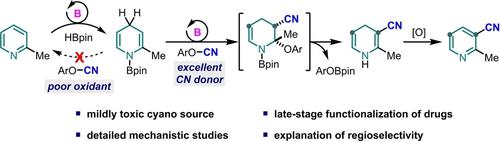
C3-selective cyanation of pyridines was accomplished by a tandem process of borane-catalyzed pyridine hydroboration, substitution of the resulting dihydropyridine with a cyano electrophile, and finally oxidative aromatization. This method was suitable for use in late-stage cyanation of pyridine drugs.
中文翻译:

吡啶的 C3-氰化:亲电子试剂的限制和区域选择性的决定因素
吡啶的 C3 选择性氰化是通过硼烷催化的吡啶硼氢化、用氰基亲电试剂取代所得二氢吡啶、最后氧化芳构化的串联过程完成的。该方法适用于吡啶类药物的后期氰化反应。
更新日期:2022-12-14
中文翻译:

吡啶的 C3-氰化:亲电子试剂的限制和区域选择性的决定因素
吡啶的 C3 选择性氰化是通过硼烷催化的吡啶硼氢化、用氰基亲电试剂取代所得二氢吡啶、最后氧化芳构化的串联过程完成的。该方法适用于吡啶类药物的后期氰化反应。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号