当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the Solid-State Electrolyte Li3+2xP1–xAlxS4: Lithium-Ion Transport Properties in Crystalline vs Glassy Phases
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-12-14 , DOI: 10.1021/acsami.2c16776
Erika P Ramos 1 , J David Bazak 2 , Abdeljalil Assoud 1 , Ashfia Huq 3 , Gillian Goward 2 , Linda F Nazar 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-12-14 , DOI: 10.1021/acsami.2c16776
Erika P Ramos 1 , J David Bazak 2 , Abdeljalil Assoud 1 , Ashfia Huq 3 , Gillian Goward 2 , Linda F Nazar 1
Affiliation
|
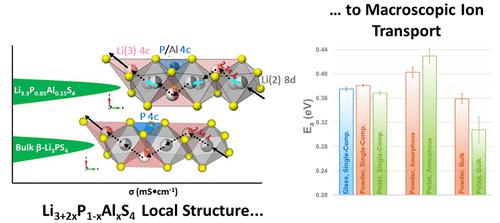
The search for new solid electrolyte materials and an understanding of fast-ion conductivity are crucial for the development of safe and high-power all-solid-state battery technology. Herein, we present the synthesis, structure, and properties of a crystalline lithium-ion conductor, Li3.3Al0.15P0.85S4 (i.e., Li9.9Al0.45P2.55S12), found in the compositional range Li3+2xP1–xAlxS4 (x = 0.15, 0.20, and 0.33). 31P magic-angle spinning nuclear magnetic resonance (MAS-NMR) aided in identifying the successful introduction of Al into the lattice. At high values of x (>0.15), crystalline Li5AlS4 and a glassy amorphous component exsolve to yield a multiphase mixture. The crystal structure of Li3.3Al0.15P0.85S4 was elucidated by single-crystal X-ray diffraction and powder neutron diffraction, demonstrating that it belongs to the thio-LISICON family with the Pnma space group, a = 12.9572(13) Å, b = 8.0861(8) Å, c = 6.1466(6) Å, and V = 644.00(11) Å3. The Li+-ion conductivity and diffusivity in this bulk material (which contains about 10 wt % of an amorphous phase, as prepared) were studied by electrochemical impedance spectroscopy and 7Li pulsed-field gradient nuclear magnetic resonance spectroscopy (PFG-NMR). The total ionic conductivity of Li3.3Al0.15P0.85S4 is 0.22(2) mS·cm–1 at room temperature with an activation energy of 0.30(1) eV. A two-component analysis method based on the Kärger equations was developed to analyze the diffusive exchange between the bulk and amorphous phases of Li3.3Al0.15P0.85S4 detected via the PFG-NMR signal attenuation curves. This approach was employed to quantitatively compare different sample morphologies (glass powder, crystalline powder, and crystalline pellets of Li3.3Al0.15P0.85S4) and assess the influence of the macroscopic state on microscopic ion transport, as supported by NMR relaxation measurements.
中文翻译:

固态电解质 Li3+2xP1–xAlxS4 的结构:结晶相与玻璃相中的锂离子传输特性
寻找新的固体电解质材料和了解快离子电导率对于开发安全和高功率的全固态电池技术至关重要。在此,我们介绍了结晶锂离子导体 Li 3.3 Al 0.15 P 0.85 S 4(即 Li 9.9 Al 0.45 P 2.55 S 12)的合成、结构和性质,其组成范围为 Li 3+2 x P 1– x Al x S 4(x = 0.15、0.20 和 0.33)。31P 魔角自旋核磁共振 (MAS-NMR) 有助于确定 Al 成功引入晶格。在高x值(>0.15) 下,结晶 Li 5 AlS 4和玻璃状无定形组分溶解产生多相混合物。通过单晶X射线衍射和粉末中子衍射阐明了Li 3.3 Al 0.15 P 0.85 S 4的晶体结构,表明它属于具有Pnma空间群的thio-LISICON家族,a = 12.9572(13) Å , b = 8.0861(8) Å, c = 6.1466(6) Å, V= 644.00(11) Å 3。通过电化学阻抗谱和7 Li 脉冲场梯度核磁共振谱 (PFG-NMR)研究了这种块状材料(其包含约 10 wt% 的非晶相,如所制备的)中的 Li +离子电导率和扩散率。室温下Li 3.3 Al 0.15 P 0.85 S 4的总离子电导率为0.22(2) mS·cm –1 ,活化能为0.30(1) eV。开发了一种基于 Kärger 方程的双组分分析方法来分析 Li 3.3 Al 0.15 P的体相和非晶相之间的扩散交换通过PFG-NMR信号衰减曲线检测到0.85 S 4 。该方法用于定量比较不同的样品形态(玻璃粉末、结晶粉末和 Li 3.3 Al 0.15 P 0.85 S 4的结晶颗粒)并评估宏观状态对微观离子传输的影响,这得到了 NMR 弛豫测量的支持。
更新日期:2022-12-14
中文翻译:

固态电解质 Li3+2xP1–xAlxS4 的结构:结晶相与玻璃相中的锂离子传输特性
寻找新的固体电解质材料和了解快离子电导率对于开发安全和高功率的全固态电池技术至关重要。在此,我们介绍了结晶锂离子导体 Li 3.3 Al 0.15 P 0.85 S 4(即 Li 9.9 Al 0.45 P 2.55 S 12)的合成、结构和性质,其组成范围为 Li 3+2 x P 1– x Al x S 4(x = 0.15、0.20 和 0.33)。31P 魔角自旋核磁共振 (MAS-NMR) 有助于确定 Al 成功引入晶格。在高x值(>0.15) 下,结晶 Li 5 AlS 4和玻璃状无定形组分溶解产生多相混合物。通过单晶X射线衍射和粉末中子衍射阐明了Li 3.3 Al 0.15 P 0.85 S 4的晶体结构,表明它属于具有Pnma空间群的thio-LISICON家族,a = 12.9572(13) Å , b = 8.0861(8) Å, c = 6.1466(6) Å, V= 644.00(11) Å 3。通过电化学阻抗谱和7 Li 脉冲场梯度核磁共振谱 (PFG-NMR)研究了这种块状材料(其包含约 10 wt% 的非晶相,如所制备的)中的 Li +离子电导率和扩散率。室温下Li 3.3 Al 0.15 P 0.85 S 4的总离子电导率为0.22(2) mS·cm –1 ,活化能为0.30(1) eV。开发了一种基于 Kärger 方程的双组分分析方法来分析 Li 3.3 Al 0.15 P的体相和非晶相之间的扩散交换通过PFG-NMR信号衰减曲线检测到0.85 S 4 。该方法用于定量比较不同的样品形态(玻璃粉末、结晶粉末和 Li 3.3 Al 0.15 P 0.85 S 4的结晶颗粒)并评估宏观状态对微观离子传输的影响,这得到了 NMR 弛豫测量的支持。

































 京公网安备 11010802027423号
京公网安备 11010802027423号