当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of Reaction Intermediates Involved in the Water Oxidation Reaction of a Molecular Cobalt Complex
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-12-14 , DOI: 10.1021/acs.inorgchem.2c03559 Moumita Bera 1 , Simarjeet Kaur 1 , Kritika Keshari 1 , Dooshaye Moonshiram 2 , Sayantan Paria 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-12-14 , DOI: 10.1021/acs.inorgchem.2c03559 Moumita Bera 1 , Simarjeet Kaur 1 , Kritika Keshari 1 , Dooshaye Moonshiram 2 , Sayantan Paria 1
Affiliation
|
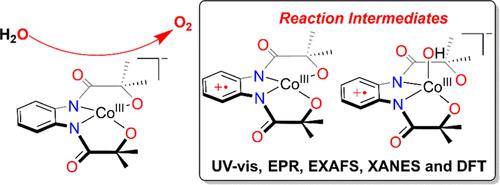
Molecular cobalt(III) complexes of bis-amidate-bis-alkoxide ligands, (Me4N)[CoIII(L1)] (1) and (Me4N)[CoIII(L2)] (2), are synthesized and assessed through a range of characterization techniques. Electrocatalytic water oxidation activity of the Co complexes in a 0.1 M phosphate buffer solution revealed a ligand-centered 2e–/1H+ transfer event at 0.99 V followed by catalytic water oxidation (WO) at an onset overpotential of 450 mV. By contrast, 2 reveals a ligand-based oxidation event at 0.9 V and a WO onset overpotential of 430 mV. Constant potential electrolysis study and rinse test experiments confirm the homogeneous nature of the Co complexes during WO. The mechanistic investigation further shows a pH-dependent change in the reaction pathway. On the one hand, below pH 7.5, two consecutive ligand-based oxidation events result in the formation of a CoIII(L2–)(OH) species, which, followed by a proton-coupled electron transfer reaction, generates a CoIV(L2–)(O) species that undergoes water nucleophilic attack to form the O–O bond. On the other hand, at higher pH, two ligand-based oxidation processes merge together and result in the formation of a CoIII(L2–)(OH) complex, which reacts with OH– to yield the O–O bond. The ligand-coordinated reaction intermediates involved in the WO reaction are thoroughly studied through an array of spectroscopic techniques, including UV–vis absorption spectroscopy, electron paramagnetic resonance, and X-ray absorption spectroscopy. A mononuclear CoIII(OH) complex supported by the one-electron oxidized ligand, [CoIII(L3–)(OH)]−, a formal CoIV(OH) complex, has been characterized, and the compound was shown to participate in the hydroxide rebound reaction, which is a functional mimic of Compound II of Cytochrome P450.
中文翻译:

参与钴分子络合物水氧化反应的反应中间体的表征
双酰胺-双醇盐配体的分子钴 (III) 配合物,(Me 4 N)[Co III (L 1 )] ( 1 ) 和 (Me 4 N)[Co III (L 2 )] ( 2 ),通过一系列表征技术进行合成和评估。Co 络合物在 0.1 M 磷酸盐缓冲溶液中的电催化水氧化活性表明在 0.99 V 时发生以配体为中心的 2e – /1H +转移事件,随后在 450 mV 的起始过电势下发生催化水氧化 (WO)。相比之下,2揭示了 0.9 V 的基于配体的氧化事件和 430 mV 的 WO 起始过电势。恒电位电解研究和漂洗测试实验证实了 WO 期间 Co 配合物的均匀性。机理研究进一步表明反应途径中的 pH 依赖性变化。一方面,在 pH 7.5 以下,两个连续的基于配体的氧化事件导致 Co III (L 2– )(OH) 物种的形成,随后发生质子耦合电子转移反应,生成 Co IV (L 2– )(O) 物种经历水亲核攻击形成 O–O 键。另一方面,在较高的 pH 值下,两个基于配体的氧化过程合并在一起并导致形成 Co III(L 2– )(OH) 络合物,与 OH –反应生成O–O 键。通过一系列光谱技术,包括紫外-可见吸收光谱、电子顺磁共振和 X 射线吸收光谱,对 WO 反应中涉及的配体配位反应中间体进行了深入研究。由单电子氧化配体 [Co III (L 3– )(OH)] −支持的单核 Co III (OH) 络合物,一种正式的 Co IV (OH) 络合物,已被表征,并且该化合物显示参与氢氧化物回弹反应,是细胞色素 P450 化合物 II 的功能模拟物。
更新日期:2022-12-14
中文翻译:

参与钴分子络合物水氧化反应的反应中间体的表征
双酰胺-双醇盐配体的分子钴 (III) 配合物,(Me 4 N)[Co III (L 1 )] ( 1 ) 和 (Me 4 N)[Co III (L 2 )] ( 2 ),通过一系列表征技术进行合成和评估。Co 络合物在 0.1 M 磷酸盐缓冲溶液中的电催化水氧化活性表明在 0.99 V 时发生以配体为中心的 2e – /1H +转移事件,随后在 450 mV 的起始过电势下发生催化水氧化 (WO)。相比之下,2揭示了 0.9 V 的基于配体的氧化事件和 430 mV 的 WO 起始过电势。恒电位电解研究和漂洗测试实验证实了 WO 期间 Co 配合物的均匀性。机理研究进一步表明反应途径中的 pH 依赖性变化。一方面,在 pH 7.5 以下,两个连续的基于配体的氧化事件导致 Co III (L 2– )(OH) 物种的形成,随后发生质子耦合电子转移反应,生成 Co IV (L 2– )(O) 物种经历水亲核攻击形成 O–O 键。另一方面,在较高的 pH 值下,两个基于配体的氧化过程合并在一起并导致形成 Co III(L 2– )(OH) 络合物,与 OH –反应生成O–O 键。通过一系列光谱技术,包括紫外-可见吸收光谱、电子顺磁共振和 X 射线吸收光谱,对 WO 反应中涉及的配体配位反应中间体进行了深入研究。由单电子氧化配体 [Co III (L 3– )(OH)] −支持的单核 Co III (OH) 络合物,一种正式的 Co IV (OH) 络合物,已被表征,并且该化合物显示参与氢氧化物回弹反应,是细胞色素 P450 化合物 II 的功能模拟物。







































 京公网安备 11010802027423号
京公网安备 11010802027423号