当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Analysis of Phosphorylation Proteoforms in a Dynamic Heterogeneous System Using Flash Oxidation Coupled In-Line with Ion Exchange Chromatography
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-12-13 , DOI: 10.1021/acs.analchem.2c04365 Zhi Cheng 1 , Sandeep K Misra 1 , Anter Shami 1 , Joshua S Sharp 1, 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-12-13 , DOI: 10.1021/acs.analchem.2c04365 Zhi Cheng 1 , Sandeep K Misra 1 , Anter Shami 1 , Joshua S Sharp 1, 2
Affiliation
|
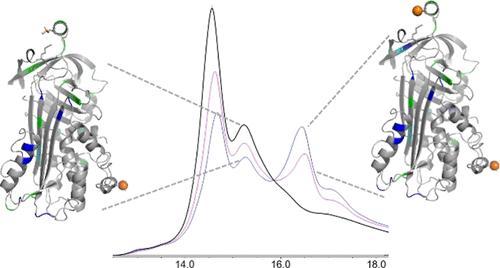
Protein posttranslational modifications (PTMs) are key modulators of protein structure and function that often change in a dynamic fashion in response to cellular stimuli. Dynamic PTMs are very challenging to structurally characterize using modern techniques, including covalent labeling methods, due to the presence of multiple proteoforms and conformers together in solution. We have coupled an ion exchange high-performance liquid chromatography separation with a flash oxidation system [ion exchange chromatography liquid chromatography-flash oxidation (IEX LC-FOX)] to successfully elucidate structural changes among three phosphoproteoforms of ovalbumin (OVA) during dephosphorylation with alkaline phosphatase. Real-time dosimetry indicates no difference in the effective radical dose between peaks or across the peak, demonstrating both the lack of scavenging of the NaCl gradient and the lack of a concentration effect on radical dose between peaks of different intensities. The use of IEX LC-FOX allows us to structurally probe into each phosphoproteoform as it elutes from the column, capturing structural data before the dynamics of the system to reintroduce heterogeneity. We found significant differences in the residue-level oxidation between the hydroxyl radical footprint of nonphosphorylated, monophosphorylated, and diphosphorylated OVA. Not only were our data consistent with the previously reported stabilization of OVA structure by phosphorylation, but local structural changes were also consistent with the measured order of dephosphorylation of Ser344 being removed first. These results demonstrate the utility of IEX LC-FOX for measuring the structural effects of PTMs, even in dynamic systems.
中文翻译:

使用闪蒸氧化与离子交换色谱在线联用对动态异质系统中的磷酸化蛋白质形式进行结构分析
蛋白质翻译后修饰 (PTM) 是蛋白质结构和功能的关键调节剂,通常会响应细胞刺激而以动态方式发生变化。由于溶液中同时存在多种蛋白质形式和构象异构体,因此使用现代技术(包括共价标记方法)对动态 PTM 进行结构表征非常具有挑战性。我们将离子交换高效液相色谱分离与闪蒸氧化系统[离子交换色谱液相色谱-闪蒸氧化 (IEX LC-FOX)] 结合起来,成功阐明了卵清蛋白 (OVA) 三种磷酸蛋白形式在碱性脱磷酸过程中的结构变化磷酸酶。实时剂量测定表明峰之间或跨峰的有效自由基剂量没有差异,这表明缺乏 NaCl 梯度的清除,并且不同强度峰之间的自由基剂量缺乏浓度效应。使用 IEX LC-FOX 使我们能够在结构上探究从柱中洗脱的每种磷酸蛋白形式,在系统动态重新引入异质性之前捕获结构数据。我们发现非磷酸化、单磷酸化和二磷酸化 OVA 的羟基自由基足迹之间的残留水平氧化存在显着差异。我们的数据不仅与之前报道的通过磷酸化稳定 OVA 结构一致,而且局部结构变化也与测量的 Ser344 去磷酸化首先被去除的顺序一致。这些结果证明了 IEX LC-FOX 在测量 PTM 结构效应方面的实用性,甚至在动态系统中也是如此。
更新日期:2022-12-13
中文翻译:

使用闪蒸氧化与离子交换色谱在线联用对动态异质系统中的磷酸化蛋白质形式进行结构分析
蛋白质翻译后修饰 (PTM) 是蛋白质结构和功能的关键调节剂,通常会响应细胞刺激而以动态方式发生变化。由于溶液中同时存在多种蛋白质形式和构象异构体,因此使用现代技术(包括共价标记方法)对动态 PTM 进行结构表征非常具有挑战性。我们将离子交换高效液相色谱分离与闪蒸氧化系统[离子交换色谱液相色谱-闪蒸氧化 (IEX LC-FOX)] 结合起来,成功阐明了卵清蛋白 (OVA) 三种磷酸蛋白形式在碱性脱磷酸过程中的结构变化磷酸酶。实时剂量测定表明峰之间或跨峰的有效自由基剂量没有差异,这表明缺乏 NaCl 梯度的清除,并且不同强度峰之间的自由基剂量缺乏浓度效应。使用 IEX LC-FOX 使我们能够在结构上探究从柱中洗脱的每种磷酸蛋白形式,在系统动态重新引入异质性之前捕获结构数据。我们发现非磷酸化、单磷酸化和二磷酸化 OVA 的羟基自由基足迹之间的残留水平氧化存在显着差异。我们的数据不仅与之前报道的通过磷酸化稳定 OVA 结构一致,而且局部结构变化也与测量的 Ser344 去磷酸化首先被去除的顺序一致。这些结果证明了 IEX LC-FOX 在测量 PTM 结构效应方面的实用性,甚至在动态系统中也是如此。















































 京公网安备 11010802027423号
京公网安备 11010802027423号