Nano Research ( IF 9.5 ) Pub Date : 2022-12-13 , DOI: 10.1007/s12274-022-5277-3
Ze-Nan Hu , Yongjian Ai , Yan Zhao , Yiming Wang , Kelong Ding , Wenhui Zhang , Rongxiu Guo , Xinyue Zhang , Xiangbin Cai , Ning Wang , Jianshe Hu , Qionglin Liang , Hongyang Liu , Fei Huang , Limin Wu , Jiangwei Zhang , Hong-bin Sun
|
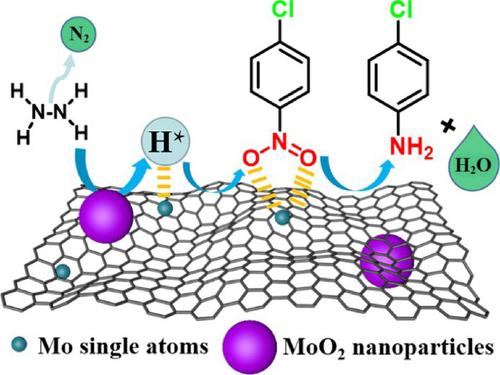
Nanocatalysts are likely to contain undetected single-atom components, which may have been ignored but have significant effect in catalytic reactions. Herein, we report a catalyst composed of Mo single atoms (SAs) and MoO2 nanoparticles (NPs) (MoSAs-MoO2@NC), which is an exact model to understand how the SAs contribute to the nanocatalyst. Both experimental results and the density functional theory calculations reveal that Mo SAs on nitrogen-doped carbon provides the reaction zone for nitro reduction, while MoO2 is the active site for decomposing hydrazine hydrate to produce H⋆. Thanks to the synergy between Mo SAs and MoO2 NPs, this catalyst exhibits noble metal-like catalytic activity (100% conversion at 4 min) for the dechlorination-proof transfer hydrogenation. Additionally, the hydrogen migration on the catalyst is verified by the electrochemical tests in the absence of a hydrogen source. This work provides a model for further study on the coexistence of single atoms in nanoparticle catalysts.
中文翻译:

确定 MoO2 纳米催化剂中 Mo 单原子组分在转移氢化中的贡献
纳米催化剂可能包含未检测到的单原子成分,这些成分可能被忽略但在催化反应中具有重要影响。在此,我们报告了一种由 Mo 单原子 (SAs) 和 MoO 2纳米颗粒 (NPs) (Mo SAs -MoO 2 @NC) 组成的催化剂,这是了解 SAs 如何对纳米催化剂做出贡献的精确模型。实验结果和密度泛函理论计算均表明,氮掺杂碳上的 Mo SAs 为硝基还原提供了反应区,而 MoO 2是分解水合肼产生 H ⋆的活性位点。由于 Mo SAs 和 MoO 2之间的协同作用NPs,该催化剂表现出类似贵金属的催化活性(4 分钟时 100% 转化率),用于防脱氯转移氢化。此外,在没有氢源的情况下,通过电化学测试验证了催化剂上的氢迁移。该工作为进一步研究单原子在纳米粒子催化剂中的共存提供了模型。







































 京公网安备 11010802027423号
京公网安备 11010802027423号