Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2022-12-13 , DOI: 10.1016/j.bmc.2022.117130 Douglas L Orsi 1 , Steven J Ferrara 1 , Stephan Siegel 2 , Anders Friberg 3 , Léa Bouché 2 , Elisabeth Pook 4 , Philip Lienau 2 , Joseph P Bluck 2 , Christopher T Lemke 1 , Gizem Akcay 5 , Timo Stellfeld 3 , Hanna Meyer 3 , Vera Pütter 3 , Simon J Holton 3 , Daniel Korr 3 , Isabel Jerchel-Furau 5 , Constantia Pantelidou 5 , Craig A Strathdee 6 , Matthew Meyerson 7 , Knut Eis 2 , Jonathan T Goldstein 6
|
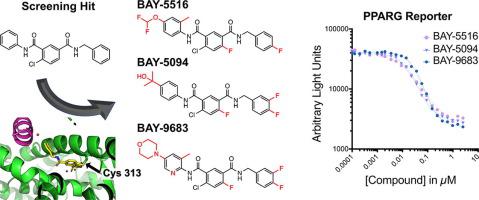
PPAR gamma (PPARG) is a ligand activated transcription factor that regulates genes involved in inflammation, bone biology, lipid homeostasis, as well as a master regulator of adipogenesis and a potential lineage driver of luminal bladder cancer. While PPARG agonists lead to transcriptional activation of canonical target genes, inverse agonists have the opposite effect through inducing a transcriptionally repressive complex leading to repression of canonical target gene expression. While many agonists have been described and tested clinically, inverse agonists offer an underexplored avenue to modulate PPARG biology in vivo. Current inverse agonists lack favorable in vivo properties; herein we describe the discovery and characterization of a series of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists, BAY-5516, BAY-5094, and BAY-9683. Structural studies of this series revealed distinct pre- and post-covalent binding positions, which led to the hypothesis that interactions in the pre-covalent conformation are primarily responsible for driving affinity, while interactions in the post-covalent conformation are more responsible for cellular functional effects by enhancing PPARG interactions with its corepressors. The need to simultaneously optimize for two distinct states may partially explain the steep SAR observed. Exquisite selectivity was achieved over related nuclear receptors in the subfamily due in part to a covalent warhead with low reactivity through an SNAr mechanism in addition to the specificity gained through covalent binding to a reactive cysteine uniquely positioned within the PPARG LBD. BAY-5516, BAY-5094, and BAY-9683 lead to pharmacodynamic regulation of PPARG target gene expression in vivo comparable to known inverse agonist SR10221 and represent new tools for future in vivo studies to explore their potential utility for treatment of disorders of hyperactivated PPARG including luminal bladder cancer and other disorders.
中文翻译:

口服生物可利用的 4-氯-6-氟间苯二胺作为共价 PPARG 反向激动剂的发现和表征
PPAR gamma (PPARG) 是一种配体激活的转录因子,可调节炎症、骨生物学、脂质稳态相关的基因,以及脂肪生成的主要调节因子和管腔膀胱癌的潜在谱系驱动因子。PPARG 激动剂导致典型靶基因的转录激活,而反向激动剂通过诱导转录抑制复合物导致典型靶基因表达抑制,从而产生相反的作用。虽然许多激动剂已在临床上得到描述和测试,但反向激动剂提供了一种尚未开发的途径来调节体内PPARG 生物学。目前的反向激动剂缺乏体内有利特性; 在此我们描述了一系列口服生物可利用的 4-氯-6-氟间苯二胺作为共价 PPARG 反向激动剂、BAY-5516、BAY-5094 和 BAY-9683 的发现和表征。该系列的结构研究揭示了不同的共价前和共价后结合位置,这导致了共价前构象中的相互作用主要负责驱动亲和力的假设,而共价后构象中的相互作用更负责细胞功能通过增强 PPARG 与其核心抑制因子的相互作用来发挥作用。同时优化两个不同状态的需要可能部分解释了观察到的陡峭 SAR。对亚家族中的相关核受体具有出色的选择性,部分原因是共价弹头通过 S 具有低反应性除了通过与 PPARG LBD 中唯一定位的反应性半胱氨酸共价结合而获得的特异性外,N Ar 机制。BAY-5516、BAY-5094 和 BAY-9683 导致体内PPARG 靶基因表达的药效学调节,与已知的反向激动剂 SR10221 相当,并代表未来体内研究的新工具,以探索其治疗过度活化 PPARG 疾病的潜在效用包括腔内膀胱癌和其他疾病。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号