当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aconitine synthesis studies. A modeling construction of the functionalized BCDE tetracyclic ring system
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-13 , DOI: 10.1039/d2qo01740g Tianyuan Guo 1 , Fuli Peng 1 , Xueqin Song 1 , Jiang Lei 1 , Fangzhou Yu 1 , Hongping Chu 1 , Keyang Yang 1 , Liang Xu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-13 , DOI: 10.1039/d2qo01740g Tianyuan Guo 1 , Fuli Peng 1 , Xueqin Song 1 , Jiang Lei 1 , Fangzhou Yu 1 , Hongping Chu 1 , Keyang Yang 1 , Liang Xu 1
Affiliation
|
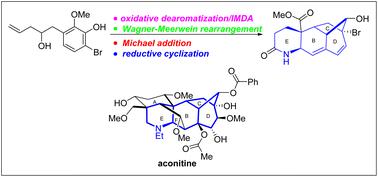
The first model synthesis of the fully functionalized BCDE tetracyclic analogue 2 of aconitine has been accomplished. A tricyclic intermediate was prepared through highly stereospecific reduction of the ketone intermediates 14 and 16 as well as the following facile Wagner–Meerwein rearrangement. A stereoselective Michael addition and reductive cyclization involving a sequential nitrile hydration and silane promoted reduction were exploited to construct the nitrogen containing six-membered E ring bearing vicinal tertiary and quaternary centers.
中文翻译:

乌头碱合成研究。功能化 BCDE 四环体系的建模构建
乌头碱全功能化 BCDE 四环类似物2的第一个模型合成已经完成。三环中间体是通过酮中间体14和16的高度立体有择还原以及随后的轻松 Wagner-Meerwein 重排制备的。利用涉及连续腈水合和硅烷促进还原的立体选择性迈克尔加成和还原环化来构建含氮的六元 E 环轴承邻三级和四级中心。
更新日期:2022-12-13
中文翻译:

乌头碱合成研究。功能化 BCDE 四环体系的建模构建
乌头碱全功能化 BCDE 四环类似物2的第一个模型合成已经完成。三环中间体是通过酮中间体14和16的高度立体有择还原以及随后的轻松 Wagner-Meerwein 重排制备的。利用涉及连续腈水合和硅烷促进还原的立体选择性迈克尔加成和还原环化来构建含氮的六元 E 环轴承邻三级和四级中心。































 京公网安备 11010802027423号
京公网安备 11010802027423号