当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS.
Nature Communications ( IF 14.7 ) Pub Date : 2016-09-15 , DOI: 10.1038/ncomms12840 Takahito Miyake , Saki Nakamura , Meng Zhao , Kanako So , Keisuke Inoue , Tomohiro Numata , Nobuaki Takahashi , Hisashi Shirakawa , Yasuo Mori , Takayuki Nakagawa , Shuji Kaneko
Nature Communications ( IF 14.7 ) Pub Date : 2016-09-15 , DOI: 10.1038/ncomms12840 Takahito Miyake , Saki Nakamura , Meng Zhao , Kanako So , Keisuke Inoue , Tomohiro Numata , Nobuaki Takahashi , Hisashi Shirakawa , Yasuo Mori , Takayuki Nakagawa , Shuji Kaneko
|
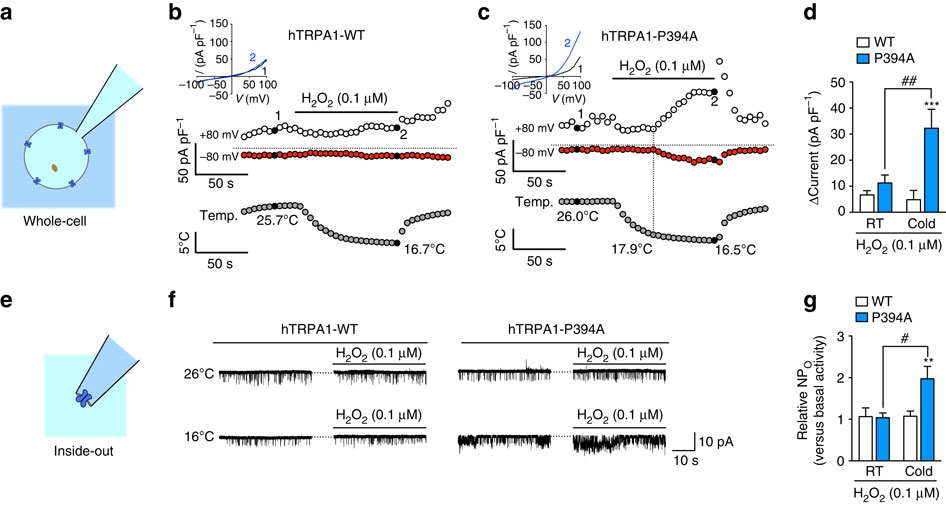
Mammalian transient receptor potential ankyrin 1 (TRPA1) is a polymodal nociceptor that plays an important role in pain generation, but its role as a cold nociceptor is still controversial. Here, we propose that TRPA1 can sense noxious cold via transduction of reactive oxygen species (ROS) signalling. We show that inhibiting hydroxylation of a proline residue within the N-terminal ankyrin repeat of human TRPA1 by mutation or using a prolyl hydroxylase (PHD) inhibitor potentiates the cold sensitivity of TRPA1 in the presence of hydrogen peroxide. Inhibiting PHD in mice triggers mouse TRPA1 sensitization sufficiently to sense cold-evoked ROS, which causes cold hypersensitivity. Furthermore, this phenomenon underlies the acute cold hypersensitivity induced by the chemotherapeutic agent oxaliplatin or its metabolite oxalate. Thus, our findings provide evidence that blocking prolyl hydroxylation reveals TRPA1 sensitization to ROS, which enables TRPA1 to convert ROS signalling into cold sensitivity.
中文翻译:

TRPA1的冷敏感性是由脯氨酰羟基化诱导的对ROS的致敏作用揭示的。
哺乳动物瞬时受体电位锚蛋白1(TRPA1)是一种多峰伤害感受器,在疼痛产生中起重要作用,但其作为冷伤害感受器的作用仍存在争议。在这里,我们建议TRPA1可以通过转导活性氧(ROS)信号来感知有害的感冒。我们表明,通过突变或使用脯氨酰羟化酶(PHD)抑制剂抑制人TRPA1的N末端锚蛋白重复序列内脯氨酸残基的羟化作用可增强TRPA1在过氧化氢存在下的冷敏感性。抑制小鼠中的PHD可充分触发小鼠TRPA1致敏,以感应冷诱发的ROS,从而引起冷超敏反应。此外,该现象是由化学治疗剂奥沙利铂或其代谢产物草酸盐引起的急性感冒超敏反应的基础。因此,
更新日期:2016-09-17
中文翻译:

TRPA1的冷敏感性是由脯氨酰羟基化诱导的对ROS的致敏作用揭示的。
哺乳动物瞬时受体电位锚蛋白1(TRPA1)是一种多峰伤害感受器,在疼痛产生中起重要作用,但其作为冷伤害感受器的作用仍存在争议。在这里,我们建议TRPA1可以通过转导活性氧(ROS)信号来感知有害的感冒。我们表明,通过突变或使用脯氨酰羟化酶(PHD)抑制剂抑制人TRPA1的N末端锚蛋白重复序列内脯氨酸残基的羟化作用可增强TRPA1在过氧化氢存在下的冷敏感性。抑制小鼠中的PHD可充分触发小鼠TRPA1致敏,以感应冷诱发的ROS,从而引起冷超敏反应。此外,该现象是由化学治疗剂奥沙利铂或其代谢产物草酸盐引起的急性感冒超敏反应的基础。因此,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号