当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclic Disaccharide Formation Enforced by a Ring Contraction: 2,3-Dideoxy Pyranoside Glycoside Donor to a Furanoside Macrocycle
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-12-09 , DOI: 10.1021/acs.joc.2c01936 Biswajit Sarkar , Titas Pramanik , Narayanaswamy Jayaraman
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-12-09 , DOI: 10.1021/acs.joc.2c01936 Biswajit Sarkar , Titas Pramanik , Narayanaswamy Jayaraman
|
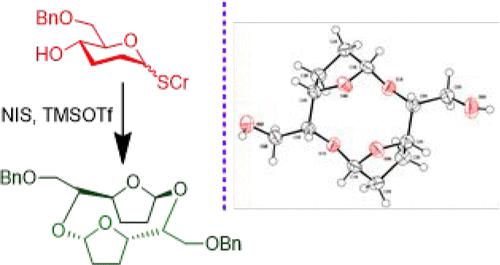
The synthesis of a disaccharide macrocycle through 2,3-dideoxy glucopyranosyl monosaccharide is reported. 2,3-Dideoxy-erythro-hexopyranosyl thioglycoside possessing a free hydroxy functionality at the C-4 carbon is prepared, and cycloglycosylation is conducted. In the event, the cycloglycosylation occurs with a ring contraction of the monosaccharide moiety and affords the cyclic furanoside disaccharide. Solution-phase and single-crystal X-ray diffraction structural characterizations permit the features of the macrocycle to be uncovered. The solubilization and encapsulation properties of the macrocycle are studied in aqueous solutions with 1-aminoadamantane.
中文翻译:

由环收缩强制形成环状二糖:2,3-双脱氧吡喃糖苷糖苷供体至呋喃糖苷大环化合物
报道了通过 2,3-二脱氧吡喃葡萄糖基单糖合成二糖大环化合物。制备在 C-4 碳原子上具有游离羟基官能团的2,3-二脱氧-赤型-己吡喃糖基硫糖苷,并进行环糖基化。在这种情况下,环糖基化随着单糖部分的环收缩而发生,并提供环状呋喃糖苷二糖。溶液相和单晶 X 射线衍射结构表征可以揭示大环的特征。在含 1-氨基金刚烷的水溶液中研究了大环化合物的增溶和包封特性。
更新日期:2022-12-09
中文翻译:

由环收缩强制形成环状二糖:2,3-双脱氧吡喃糖苷糖苷供体至呋喃糖苷大环化合物
报道了通过 2,3-二脱氧吡喃葡萄糖基单糖合成二糖大环化合物。制备在 C-4 碳原子上具有游离羟基官能团的2,3-二脱氧-赤型-己吡喃糖基硫糖苷,并进行环糖基化。在这种情况下,环糖基化随着单糖部分的环收缩而发生,并提供环状呋喃糖苷二糖。溶液相和单晶 X 射线衍射结构表征可以揭示大环的特征。在含 1-氨基金刚烷的水溶液中研究了大环化合物的增溶和包封特性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号