当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonflammable Dual-Salt Electrolytes for Graphite/LiNi0.8Co0.1Mn0.1O2 Lithium-Ion Batteries: Li+ Solvation Structure and Electrode/Eelectrolyte Interphase
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-12-05 , DOI: 10.1021/acsaem.2c03065
Fanjue Wen 1 , Shuai Cao 1 , Xin Ren 1 , Yuliang Cao 2 , Xinping Ai 2 , Fei Xu 1, 3
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-12-05 , DOI: 10.1021/acsaem.2c03065
Fanjue Wen 1 , Shuai Cao 1 , Xin Ren 1 , Yuliang Cao 2 , Xinping Ai 2 , Fei Xu 1, 3
Affiliation
|
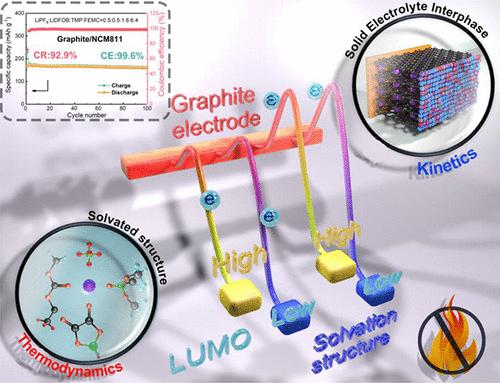
Trimethyl phosphate (TMP) is the most promising safe solvent for lithium-ion battery (LIB) electrolyte because of the nonflammability, oxidation stability, and low cost, but its application is hindered by incompatibility with the graphite anode. Herein, nonflammable electrolytes with ordinary concentration (1 mol L–1) are designed for graphite/LiNi0.8Co0.1Mn0.1O2 (Gr/NCM811) LIBs with TMP/2,2,2-trifluoroethyl methyl carbonate (FEMC) binary solvents. Stable cycling of the Li/Gr half cells with high capacity is achieved via modulation of the Li+ solvation structure. A dual-salt strategy of lithium hexafluorophosphate/lithium difluoro(oxalato)borate is further used to realize the high performance of the Gr/NCM811 full cells. More significantly, the functions and relationship of Li+ solvation structure and electrode/electrolyte interphase are elucidated. Li+ solvation structure and interphase are respectively the thermodynamic and kinetic factors for the side reactions of the electrolyte occurring at the electrode/electrolyte interphase, which should be considered comprehensively in the design of electrolytes for high-energy density LIBs.
中文翻译:

用于石墨/LiNi0.8Co0.1Mn0.1O2 锂离子电池的不可燃双盐电解质:Li+ 溶剂化结构和电极/电解质界面
磷酸三甲酯(TMP)具有不可燃性、氧化稳定性和低成本等优点,是锂离子电池(LIB)电解液最有前途的安全溶剂,但由于与石墨负极不相容,阻碍了其应用。在此,具有普通浓度 (1 mol L –1 ) 的不可燃电解质设计用于石墨/LiNi 0.8 Co 0.1 Mn 0.1 O 2 (Gr/NCM811) LIBs 与 TMP/2,2,2-三氟甲基碳酸甲酯 (FEMC) 二元溶剂. 通过调节 Li +实现高容量 Li/Gr 半电池的稳定循环溶剂化结构。六氟磷酸锂/二氟(草酸)硼酸锂的双盐策略进一步用于实现 Gr/NCM811 全电池的高性能。更重要的是,阐明了 Li +溶剂化结构和电极/电解质界面的功能和关系。Li +溶剂化结构和界面分别是电解质在电极/电解质界面发生副反应的热力学和动力学因素,在设计高能量密度锂离子电池电解质时应综合考虑。
更新日期:2022-12-05
中文翻译:

用于石墨/LiNi0.8Co0.1Mn0.1O2 锂离子电池的不可燃双盐电解质:Li+ 溶剂化结构和电极/电解质界面
磷酸三甲酯(TMP)具有不可燃性、氧化稳定性和低成本等优点,是锂离子电池(LIB)电解液最有前途的安全溶剂,但由于与石墨负极不相容,阻碍了其应用。在此,具有普通浓度 (1 mol L –1 ) 的不可燃电解质设计用于石墨/LiNi 0.8 Co 0.1 Mn 0.1 O 2 (Gr/NCM811) LIBs 与 TMP/2,2,2-三氟甲基碳酸甲酯 (FEMC) 二元溶剂. 通过调节 Li +实现高容量 Li/Gr 半电池的稳定循环溶剂化结构。六氟磷酸锂/二氟(草酸)硼酸锂的双盐策略进一步用于实现 Gr/NCM811 全电池的高性能。更重要的是,阐明了 Li +溶剂化结构和电极/电解质界面的功能和关系。Li +溶剂化结构和界面分别是电解质在电极/电解质界面发生副反应的热力学和动力学因素,在设计高能量密度锂离子电池电解质时应综合考虑。































 京公网安备 11010802027423号
京公网安备 11010802027423号