当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Site Model for Ab Initio Calculations of Gibbs Free Energies and Enthalpies of Adsorption: Methane in Zeolite Mobile Five (H-MFI)
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2022-12-08 , DOI: 10.1021/acs.jpclett.2c03302
Marcin Rybicki 1 , Kaido Sillar 1, 2 , Joachim Sauer 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2022-12-08 , DOI: 10.1021/acs.jpclett.2c03302
Marcin Rybicki 1 , Kaido Sillar 1, 2 , Joachim Sauer 1
Affiliation
|
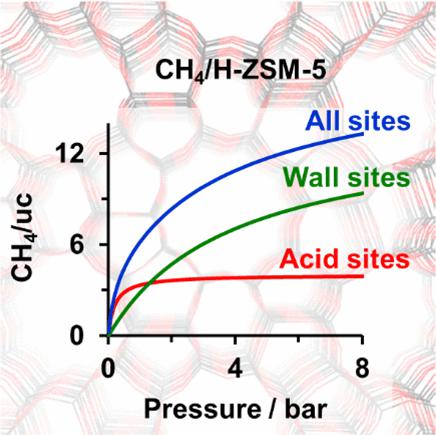
Quantum chemical hybrid MP2:PBE+D2 calculations in combination with molecular statistics are employed to calculate enthalpies and Gibbs free energies of adsorption for CH4 at Brønsted acid sites [bridging Si–O(H)–Al groups] and silica wall sites (Si–O–Si) of the proton form of zeolite MFI (H-ZSM-5) and its purely siliceous analogue Silicalite-1. A Langmuir model is adopted to calculate the amounts of CH4 adsorbed at each type of site from the Gibbs free energies. The combination of these results according to the ratio of silica wall sites and Brønsted acid sites in the sample yields adsorption isotherms for zeolites with different Si/Al ratios. The zero-coverage isosteric heats of adsorption, calculated as thermal averages of the adsorption enthalpies of the individual sites, vary between 20.2 kJ/mol for the pore wall site and 29.2 kJ/mol for the acid site and agree well within ±1 kJ/mol with experimental results.
中文翻译:

吉布斯自由能和吸附焓的从头算计算的双站点模型:沸石流动五 (H-MFI) 中的甲烷
量子化学杂化 MP2:PBE+D2 计算与分子统计相结合,用于计算 CH 4在 Brønsted 酸位点 [桥接 Si–O(H)–Al 基团] 和硅壁位点 (Si –O–Si) 质子形式的沸石 MFI (H-ZSM-5) 及其纯硅质类似物 Silicalite-1。采用Langmuir模型计算CH 4的量从吉布斯自由能吸附在每种类型的位置。根据样品中二氧化硅壁位点和 Brønsted 酸位点的比率,这些结果的组合产生了具有不同 Si/Al 比率的沸石的吸附等温线。零覆盖等量吸附热(计算为各个位点的吸附焓的热平均值)在孔壁位点的 20.2 kJ/mol 和酸性位点的 29.2 kJ/mol 之间变化,并且在 ±1 kJ/mol 范围内很好地吻合摩尔与实验结果。
更新日期:2022-12-08
中文翻译:

吉布斯自由能和吸附焓的从头算计算的双站点模型:沸石流动五 (H-MFI) 中的甲烷
量子化学杂化 MP2:PBE+D2 计算与分子统计相结合,用于计算 CH 4在 Brønsted 酸位点 [桥接 Si–O(H)–Al 基团] 和硅壁位点 (Si –O–Si) 质子形式的沸石 MFI (H-ZSM-5) 及其纯硅质类似物 Silicalite-1。采用Langmuir模型计算CH 4的量从吉布斯自由能吸附在每种类型的位置。根据样品中二氧化硅壁位点和 Brønsted 酸位点的比率,这些结果的组合产生了具有不同 Si/Al 比率的沸石的吸附等温线。零覆盖等量吸附热(计算为各个位点的吸附焓的热平均值)在孔壁位点的 20.2 kJ/mol 和酸性位点的 29.2 kJ/mol 之间变化,并且在 ±1 kJ/mol 范围内很好地吻合摩尔与实验结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号