当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Voltammetric Study and Modeling of the Electrochemical Oxidation Process and the Adsorption Effects of Luminol and Luminol Derivatives on Glassy Carbon Electrodes
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-12-08 , DOI: 10.1021/acs.analchem.2c04297 Ran Chen 1 , Xin Wang 1 , Kaiqing Wu 1 , Songqin Liu 1 , Yuanjian Zhang 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-12-08 , DOI: 10.1021/acs.analchem.2c04297 Ran Chen 1 , Xin Wang 1 , Kaiqing Wu 1 , Songqin Liu 1 , Yuanjian Zhang 1
Affiliation
|
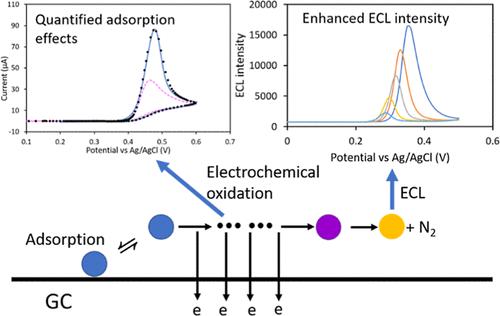
Luminol is one of the most widely used electrochemiluminescence (ECL) reagents, yet the detailed mechanism and kinetics of the electrochemical oxidation of luminol remain unclear. We propose a model that describes the electrochemical oxidation of luminol as multiple electron transfer reactions followed by an irreversible chemical reaction, and we applied a finite element method simulation to analyze the electron transfer kinetics in alkaline solutions. Although negligible at higher pH values, the adsorption of luminol on the glassy carbon electrode became noticeable in a solution with pH = 12. Additionally, various types of adsorption behaviors were observed for luminol derivatives and analogues, indicating that the molecular structure affected not only the oxidation but also the adsorption process. The adsorption effect was analyzed through a model with a Langmuir isotherm to show that the saturated surface concentration as well as the reaction kinetics increased with decreasing pH, suggesting a competition for the active sites between the molecule and OH–. Moreover, we show that the ECL intensity could be boosted through the adsorption effect by collecting the ECL intensity generated through the electrochemical oxidation of luminol and a luminol analogue, L012, in a solution with pH = 13. In contrast with luminol, a significant adsorption effect was observed for L012 at pH = 13, and the ECL intensity was enhanced by the adsorbed species, especially at higher scan rates. The magnitude of the enhancement of the ECL intensity matched well with the simulation using our model.
中文翻译:

电化学氧化过程的伏安法研究和建模以及鲁米诺和鲁米诺衍生物对玻碳电极的吸附作用
鲁米诺是使用最广泛的电化学发光 (ECL) 试剂之一,但鲁米诺电化学氧化的详细机制和动力学仍不清楚。我们提出了一个模型,将鲁米诺的电化学氧化描述为多次电子转移反应,然后是不可逆化学反应,并且我们应用有限元法模拟来分析碱性溶液中的电子转移动力学。虽然在较高的 pH 值下可以忽略不计,但在 pH = 12 的溶液中,鲁米诺在玻碳电极上的吸附变得明显。此外,观察到鲁米诺衍生物和类似物的各种类型的吸附行为,表明分子结构不仅影响氧化也是吸附过程。–。此外,我们表明,通过收集鲁米诺和鲁米诺类似物 L012 在 pH = 13 的溶液中电化学氧化产生的 ECL 强度,可以通过吸附效应提高 ECL 强度。与鲁米诺相比,显着的吸附在 pH = 13 时观察到 L012 的效果,并且 ECL 强度被吸附的物质增强,特别是在较高的扫描速率下。ECL 强度增强的幅度与使用我们的模型进行的模拟非常吻合。
更新日期:2022-12-08
中文翻译:

电化学氧化过程的伏安法研究和建模以及鲁米诺和鲁米诺衍生物对玻碳电极的吸附作用
鲁米诺是使用最广泛的电化学发光 (ECL) 试剂之一,但鲁米诺电化学氧化的详细机制和动力学仍不清楚。我们提出了一个模型,将鲁米诺的电化学氧化描述为多次电子转移反应,然后是不可逆化学反应,并且我们应用有限元法模拟来分析碱性溶液中的电子转移动力学。虽然在较高的 pH 值下可以忽略不计,但在 pH = 12 的溶液中,鲁米诺在玻碳电极上的吸附变得明显。此外,观察到鲁米诺衍生物和类似物的各种类型的吸附行为,表明分子结构不仅影响氧化也是吸附过程。–。此外,我们表明,通过收集鲁米诺和鲁米诺类似物 L012 在 pH = 13 的溶液中电化学氧化产生的 ECL 强度,可以通过吸附效应提高 ECL 强度。与鲁米诺相比,显着的吸附在 pH = 13 时观察到 L012 的效果,并且 ECL 强度被吸附的物质增强,特别是在较高的扫描速率下。ECL 强度增强的幅度与使用我们的模型进行的模拟非常吻合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号