Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-12-06 , DOI: 10.1016/j.apcatb.2022.122272 Xiangzhou Lv , Qian Liu , Jianghao Wang , Xiuju Wu , Xiaotong Li , Yue Yang , Jianhua Yan , Angjian Wu , Hao Bin Wu
|
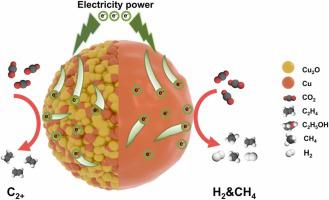
The oxidation status of Cu-based materials have been proved to be essential to the catalytical performances of electrochemical CO2 reduction. The coexistence of Cu+ and Cu0 species is generally considered as the origin of superior catalytic performance, yet the Cu+ moieties are subject to reduction under negative potentials especially at high current density. In this work, we report a grain refining approach to tune the oxidation states of Cu-based catalysts by modulating the electron transfer during electrochemical CO2 reduction reaction (CO2RR) process when the in-situ electroreduction of Cu+ species occurs. Cu2O nanospheres with abundant grain boundaries exhibited lower electron conductivity compared with Cu2O nanospheres with less grain boundaries, which can hinder the complete reduction of Cu2O and maintain Cu+ species under high current densities. As a result, the multi-grain Cu2O showed a maximum FE of ∼79% for C2+ products at a high current density of 800 mA cm−2, notably surpassing the later. Experimental and theoretical analyses indicated that mixed Cu+/Cu0 states of multi-grain Cu2O during reaction, favoring the C-C coupling process towards C2+ products. This work demonstrates the feasibility to tune the real valence state of catalytic sites under operational conditions by nanostructure engineering.
中文翻译:

晶粒细化使混合 Cu+/Cu0 状态能够在高电流密度下将 CO2 电还原为 C2+ 产品
铜基材料的氧化状态已被证明对电化学CO 2还原的催化性能至关重要。Cu +和Cu 0物种的共存通常被认为是优异催化性能的来源,但Cu +部分在负电位下特别是在高电流密度下容易被还原。在这项工作中,我们报告了一种晶粒细化方法,通过在 Cu +物种发生原位电还原时调节电化学 CO 2还原反应 (CO 2 RR) 过程中的电子转移来调节 Cu 基催化剂的氧化态。铜2与晶界较少的 Cu 2 O 纳米球相比,晶界丰富的 O 纳米球表现出较低的电子传导性,这会阻碍 Cu 2 O 的完全还原并在高电流密度下保持 Cu +物种。结果,在800 mA cm -2的高电流密度下,多晶 Cu 2 O 对于 C 2+产物显示出最大 FE 约为 79% ,明显超过了后者。实验和理论分析表明,反应过程中多晶Cu 2 O的混合Cu + /Cu 0态有利于CC偶联过程向C 2+的方向发展产品。这项工作证明了通过纳米结构工程在操作条件下调整催化位点的真实价态的可行性。































 京公网安备 11010802027423号
京公网安备 11010802027423号