当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Electrophilic α-Alkynylation of α,α-Disubstituted N-tert-Butanesulfinyl Ketimines for Construction of Less Accessible Acyclic Quaternary Stereocenters
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-07 , DOI: 10.1021/acs.orglett.2c03865
Li-Feng Chu 1 , Yun Yao 1 , Chong-Dao Lu 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-07 , DOI: 10.1021/acs.orglett.2c03865
Li-Feng Chu 1 , Yun Yao 1 , Chong-Dao Lu 1
Affiliation
|
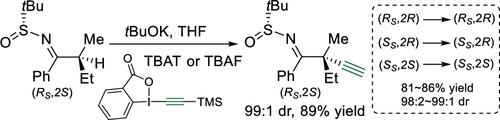
In order to construct less accessible acyclic quaternary stereocenters, a protocol was developed to α-alkynylate α,α-disubstituted N-tert-butanesulfinyl ketimines stereoselectively using 1-(2-trimethylsilylethynyl)-1,2-benziodoxol-3(1H)-one in the presence of fluoride. Despite the steric and electrical similarity between the two α-substituents, the entire reaction proceeded in a strongly stereoselective manner: tBuOK promoted α-deprotonation of the acyclic ketimine to generate stereodefined fully substituted aza-enolates, which stereoselectively formed C–C bonds with electrophilic alkynylation reagents, affording α-alkynylation products with excellent stereocontrol.
中文翻译:

α,α-二取代的 N-叔丁基亚磺酰基酮亚胺的立体选择性亲电 α-炔基化,用于构建不易接近的无环季立体中心
为了构建不易接近的无环季立体中心,开发了一种方案,使用 1-(2-trimethylsilylethynyl)-1,2-benziodoxol-3(1 H )对 α-炔基化 α,α-二取代的N -叔丁亚磺酰基酮亚胺进行立体选择性- 一个在氟化物存在的情况下。尽管两个 α- 取代基之间存在空间和电学相似性,但整个反应以强烈的立体选择性方式进行:t BuOK 促进无环酮亚胺的 α- 去质子化以生成立体定义的完全取代的氮杂烯醇化物,其立体选择性地与亲电炔基化试剂,提供具有优良立体控制的α-炔基化产物。
更新日期:2022-12-07
中文翻译:

α,α-二取代的 N-叔丁基亚磺酰基酮亚胺的立体选择性亲电 α-炔基化,用于构建不易接近的无环季立体中心
为了构建不易接近的无环季立体中心,开发了一种方案,使用 1-(2-trimethylsilylethynyl)-1,2-benziodoxol-3(1 H )对 α-炔基化 α,α-二取代的N -叔丁亚磺酰基酮亚胺进行立体选择性- 一个在氟化物存在的情况下。尽管两个 α- 取代基之间存在空间和电学相似性,但整个反应以强烈的立体选择性方式进行:t BuOK 促进无环酮亚胺的 α- 去质子化以生成立体定义的完全取代的氮杂烯醇化物,其立体选择性地与亲电炔基化试剂,提供具有优良立体控制的α-炔基化产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号