European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-12-06 , DOI: 10.1016/j.ejmech.2022.114998 Jimin Xu 1 , Shuizhen Shi 2 , Gang Liu 1 , Xuping Xie 3 , Jun Li 1 , Andrew A Bolinger 1 , Haiying Chen 1 , Wenbo Zhang 2 , Pei-Yong Shi 3 , Hua Liu 2 , Jia Zhou 1
|
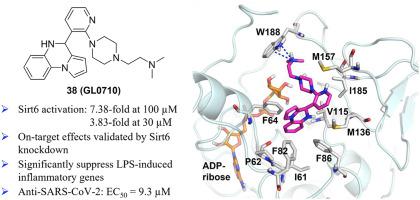
Sirt6 activation has emerged as a promising drug target for the treatment of various human diseases, while only limited Sirt6 activators have been reported. Herein, a series of novel pyrrolo[1,2-a]quinoxaline-based derivatives have been identified as potent and selective Sirt6 activators with low cytotoxicity. Sirt6-knockdown findings have validated the on-target effects of this class of Sirt6 activators. Docking studies indicate the protonated nitrogen on the side chain of 38 forms π-cation interactions with Trp188, further stabilizing it into this extended binding pocket. New compounds 35, 36, 38, 46, 47, and 50 strongly repressed LPS-induced proinflammatory cytokine/chemokine production, while 38 also significantly suppressed SARS-CoV-2 infection with an EC50 value of 9.3 μM. Moreover, compound 36 significantly inhibited the colony formation of cancer cells. These new molecules may serve as useful pharmacological tools or potential therapeutics against cancer, inflammation, and infectious diseases.
中文翻译:

作为有效和选择性 Sirt6 激活剂的吡咯并[1,2-a]喹喔啉基衍生物的设计、合成和药理学评价
Sirt6 激活已成为治疗各种人类疾病的有前景的药物靶点,但目前仅报道了有限的 Sirt6 激活剂。在此,一系列新型吡咯并[1,2- a ]喹喔啉衍生物已被鉴定为具有低细胞毒性的有效、选择性Sirt6激活剂。 Sirt6 敲低研究结果验证了此类 Sirt6 激活剂的靶向作用。对接研究表明, 38侧链上的质子化氮与 Trp188 形成 π 阳离子相互作用,进一步将其稳定在这个扩展的结合袋中。新化合物35、36、38、46、47和50强烈抑制 LPS 诱导的促炎细胞因子/趋化因子的产生,而38也显着抑制 SARS - CoV -2 感染,EC 50值为 9.3 μM。此外,化合物36显着抑制癌细胞集落形成。这些新分子可以作为有用的药理学工具或针对癌症、炎症和传染病的潜在疗法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号