当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Scalable Route for a Key Benzothiazole Building Block via a Pd-Catalyzed Migita Coupling with a Nonsmelly Thiol Surrogate
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-12-06 , DOI: 10.1021/acs.oprd.2c00331 Gabriel Schäfer 1 , Aurélien Merot 1 , Tony Fleischer 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-12-06 , DOI: 10.1021/acs.oprd.2c00331 Gabriel Schäfer 1 , Aurélien Merot 1 , Tony Fleischer 1
Affiliation
|
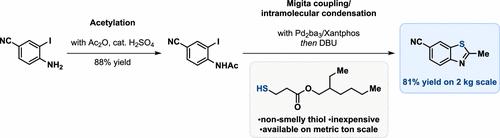
2-Methylbenzo[d]thiazole-6-carbonitrile was internally identified as an important building block and therefore, kg amounts of it needed to be urgently supplied. As the desired benzothiazole was only available in small quantities for a high price (∼1000 USD/g), a robust and scalable route needed to be rapidly developed. The key to success was the use of a 2-iodophenyl N-acetamide precursor to construct the 2-methylbenzo[d]thiazole core via a Pd-catalyzed Migita coupling followed by subsequent intramolecular condensation of the intermediate thiophenol onto the N-acetyl group. To avoid the use of malodorous thiols on scale, 2-ethylhexyl 3-mercaptopropionate was used as a nonsmelly, inexpensive thiol surrogate. The Migita coupling was extensively optimized and allowed the reaction to be performed in a dose-controlled manner with regard to the addition of the thiol surrogate at 40 °C, by using only 0.1 mol % Pd2dba3. The subsequent intramolecular cyclization step was promoted by the one-pot addition of DBU. After aqueous workup and crystallization, 2-methylbenzo[d]thiazole-6-carbonitrile was obtained in 81% yield and excellent purity (99.7% a/a) on a 2.0 kg scale.
中文翻译:

通过 Pd 催化的 Migita 偶联与无臭硫醇替代物开发关键苯并噻唑结构单元的可扩展路线
2-甲基苯并[ d ]噻唑-6-腈被内部确定为重要的组成部分,因此需要紧急供应 kg 数量。由于所需的苯并噻唑只能以高价(~1000 美元/克)少量获得,因此需要快速开发稳健且可扩展的路线。成功的关键是使用 2-碘苯基N-乙酰胺前体通过 Pd 催化的 Migita 偶联构建 2-甲基苯并 [ d ] 噻唑核,随后将中间体苯硫酚分子内缩合到N-乙酰基。为避免在规模上使用恶臭硫醇,使用 2-乙基己基 3-巯基丙酸酯作为无味、廉价的硫醇替代物。通过仅使用 0.1 mol% Pd 2 dba 3,Migita 偶联得到了广泛优化,并允许在 40 °C 下以剂量控制的方式进行反应,以添加硫醇替代物。通过一锅法添加 DBU 促进了随后的分子内环化步骤。在水处理和结晶后,2-甲基苯并[ d ]噻唑-6-腈以 81% 的产率和极好的纯度 (99.7% a/a) 获得,规模为 2.0 千克。
更新日期:2022-12-06
中文翻译:

通过 Pd 催化的 Migita 偶联与无臭硫醇替代物开发关键苯并噻唑结构单元的可扩展路线
2-甲基苯并[ d ]噻唑-6-腈被内部确定为重要的组成部分,因此需要紧急供应 kg 数量。由于所需的苯并噻唑只能以高价(~1000 美元/克)少量获得,因此需要快速开发稳健且可扩展的路线。成功的关键是使用 2-碘苯基N-乙酰胺前体通过 Pd 催化的 Migita 偶联构建 2-甲基苯并 [ d ] 噻唑核,随后将中间体苯硫酚分子内缩合到N-乙酰基。为避免在规模上使用恶臭硫醇,使用 2-乙基己基 3-巯基丙酸酯作为无味、廉价的硫醇替代物。通过仅使用 0.1 mol% Pd 2 dba 3,Migita 偶联得到了广泛优化,并允许在 40 °C 下以剂量控制的方式进行反应,以添加硫醇替代物。通过一锅法添加 DBU 促进了随后的分子内环化步骤。在水处理和结晶后,2-甲基苯并[ d ]噻唑-6-腈以 81% 的产率和极好的纯度 (99.7% a/a) 获得,规模为 2.0 千克。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号