当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hexachloro-1,3-butadiene as a Functional Additive for Constructing an Efficient Solid Electrolyte Interface Layer for Long-Life Stable Li Anodes
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-12-06 , DOI: 10.1021/acsami.2c18783 Xiangxiang Fu 1 , Huanhuan Duan 1 , Shiwei Zhang 1 , Ran Bi 1 , Yuanfu Deng 1, 2 , Guohua Chen 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-12-06 , DOI: 10.1021/acsami.2c18783 Xiangxiang Fu 1 , Huanhuan Duan 1 , Shiwei Zhang 1 , Ran Bi 1 , Yuanfu Deng 1, 2 , Guohua Chen 3
Affiliation
|
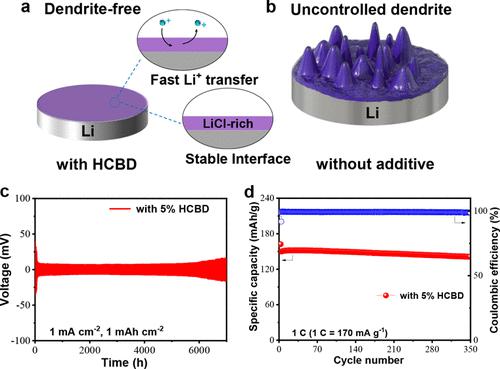
Lithium (Li) metal is considered as one of the attractive anodes for next-generation high-energy-density batteries due to its ultrahigh theoretical specific capacity and low potential. However, many great challenges including uncontrolled dendrite growth and undesired side reactions during repeated cycling still seriously hinder its practical application in Li metal secondary batteries. Herein, we report the hexachloro-1,3-butadiene (HCBD) molecule as a functional additive to stabilize the Li anode by forming a stable solid electrolyte interface (SEI) layer with high Li ion conductivity via in situ surface and electrochemical reactions. Density functional theory calculations demonstrate that HCBD can preferentially react with the Li anode, which generates an ionic conducting species (LiCl) into an SEI layer. The LiCl-rich SEI layer effectively regulates Li+ deposition/stripping kinetics and then induces uniform nucleation of Li+ and reduces the side reactions between the Li anode and electrolyte. With an optimal amount of HCBD in an ether-based electrolyte, an excellent cycling lifespan (7000 h) was achieved with a low hysteresis voltage of ∼10 mV at 1.0 mA cm–2 in a Li||Li symmetrical cell. Furthermore, the LiFePO4-based cell with the additive-functionalized Li anode displays obviously improved cycling stability (with a high specific capacity of 141.1 mAh g–1 after 350 cycles at 1 C).
中文翻译:

六氯-1,3-丁二烯作为功能性添加剂构建长寿命稳定锂负极的高效固体电解质界面层
由于其超高的理论比容量和低电位,锂 (Li) 金属被认为是下一代高能量密度电池的有吸引力的负极之一。然而,许多巨大的挑战,包括不受控制的枝晶生长和重复循环过程中不希望发生的副反应,仍然严重阻碍了其在锂金属二次电池中的实际应用。在此,我们报道了六氯-1,3-丁二烯 (HCBD) 分子作为一种功能性添加剂,通过原位表面和电化学反应形成具有高锂离子电导率的稳定固体电解质界面 (SEI) 层,从而稳定锂负极。密度泛函理论计算表明,六氯丁二烯可以优先与锂负极发生反应,从而在 SEI 层中生成离子导电物质 (LiCl)。+沉积/剥离动力学,然后诱导 Li +的均匀成核并减少 Li 负极和电解质之间的副反应。在醚基电解质中加入最佳量的六氯丁二烯后,Li||Li 对称电池在 1.0 mA cm –2下的低滞后电压为 ~10 mV,实现了出色的循环寿命(7000 小时)。此外,具有添加剂功能化锂负极的基于LiFePO 4的电池显示出明显改善的循环稳定性(在 1 C 下循环 350 次后具有 141.1 mAh g –1的高比容量)。
更新日期:2022-12-06
中文翻译:

六氯-1,3-丁二烯作为功能性添加剂构建长寿命稳定锂负极的高效固体电解质界面层
由于其超高的理论比容量和低电位,锂 (Li) 金属被认为是下一代高能量密度电池的有吸引力的负极之一。然而,许多巨大的挑战,包括不受控制的枝晶生长和重复循环过程中不希望发生的副反应,仍然严重阻碍了其在锂金属二次电池中的实际应用。在此,我们报道了六氯-1,3-丁二烯 (HCBD) 分子作为一种功能性添加剂,通过原位表面和电化学反应形成具有高锂离子电导率的稳定固体电解质界面 (SEI) 层,从而稳定锂负极。密度泛函理论计算表明,六氯丁二烯可以优先与锂负极发生反应,从而在 SEI 层中生成离子导电物质 (LiCl)。+沉积/剥离动力学,然后诱导 Li +的均匀成核并减少 Li 负极和电解质之间的副反应。在醚基电解质中加入最佳量的六氯丁二烯后,Li||Li 对称电池在 1.0 mA cm –2下的低滞后电压为 ~10 mV,实现了出色的循环寿命(7000 小时)。此外,具有添加剂功能化锂负极的基于LiFePO 4的电池显示出明显改善的循环稳定性(在 1 C 下循环 350 次后具有 141.1 mAh g –1的高比容量)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号